Clinical impact of human papillomavirus in laryngeal squamous cell carcinoma: a retrospective study
- Published
- Accepted
- Received
- Academic Editor
- Philip Coates
- Subject Areas
- Oncology, Otorhinolaryngology, Pathology
- Keywords
- HPV, Laryngeal cancer, Survival, Recurrence, Prevalence
- Copyright
- © 2017 Chen et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2017. Clinical impact of human papillomavirus in laryngeal squamous cell carcinoma: a retrospective study. PeerJ 5:e3395 https://doi.org/10.7717/peerj.3395
Abstract
Objectives
The purpose of this study is to determine the prevalence and clinical impact of human papillomavirus (HPV) related laryngeal squamous cell carcinoma (LSCC).
Methods
A total of 106 LSCC patients who underwent primary surgical resection with or without adjuvant radiotherapy/chemoradiotherapy were enrolled retrospectively. Tumors collected from paraffin-embedded samples were used for HPV detection by polymerase chain reaction and in situ hybridization technique. Clinicopathological parameters were recorded for analysis.
Results
The prevalence of HPV in patients with LSCC was 13.2% in our series and 12 out of 14 (85.7%) HPV-positive tumors were HPV-16. The patients with HPV-positive tumors were older (p = 0.042), less local/regional recurrence (p = 0.037) and non-smoker (p = 0.068). There was no significant difference in the 5-year overall survival (OS) (p = 0.8056) between HPV-positive and -negative tumors. The patients with HPV-positive tumors had a better 5-year disease-specific survival (DSS) (100% vs. 84.8%, p = 0.1485), although the difference did not reach statistical significance. However, the local/regional control rate was significantly better in HPV-positive tumors than in HPV-negative tumors (100% vs. 75%, p = 0.0494).
Conclusions
A low prevalence of HPV infection in our series suggests that HPV is not a major cause of LSCC. However, a 100% local/regional control rate and DSS were observed in HPV-positive tumors. This finding suggests a different tumor behavior between HPV-positive and HPV-negative LSCC. Further research with a larger sample size is necessary to confirm our observations.
Introduction
The high-oncogenic risk types of human papillomavirus (HPV) can induce tumorigenesis via the E6 and E7 viral oncoproteins. These oncoproteins can functionally inactivate the tumor suppressor proteins p53 and pRb, resulting in a loss of cell cycle regulation and immortalization of keratinocytes (Havre et al., 1995; Munger & Howley, 2002). HPV-associated cancers are well documented in cervical cancer, in which 99.7% of cases harbor a high-risk HPV type (Walboomers et al., 1999). At the end of the last century, an association between HPV and head and neck squamous cell carcinoma (HNSCC) was identified, with an overall prevalence of 25% of tumors harboring HPV (Hoffmann et al., 1998; McKaig, Baric & Olshan, 1998; Kreimer et al., 2005). There is mounting evidence of a strong association between HPV and oropharyngeal squamous cell carcinoma (OPSCC), as documented in Europe and the United States (Ang et al., 2010). Currently, HPV-positive and HPV-negative OPSCCs are thought to be two distinct diseases.
Previously, we found that Taiwanese patients with oropharyngeal cancer had a lower prevalence of HPV than that of patients from Western populations (Chien et al., 2008; Armas et al., 2008). In Taiwan, betel nut chewing has a significant impact on health and may cause these differences. Betel nut chewing plays an important role in the development of upper aerodigestive tract malignancies, and synchronous or metachronous tumors of the upper aerodigestive tract are commonly observed in these patients (Su et al., 2013). Lee et al. (2005) also observed that betel nut chewing and tobacco have a synergistic effect on the development of LSCC.
The microenvironment of the laryngeal mucosa is similar to that of the uterine cervix, which has an epithelial junctional area between squamous and columnar epithelia; the junctional area is a potential site for HPV infection (Koskinen et al., 2007). Previous studies have found that low-risk HPV is associated with recurrent respiratory papillomatosis (Bonagura et al., 2010), while high-risk HPV is associated with laryngeal cancer (De Oliveira et al., 2006; Ma et al., 1998; Morshed, 2010). However, the association between laryngeal squamous cell carcinoma (LSCC) and HPV infection remains controversial due to inconsistent results (Lee et al., 2011; Upile et al., 2014; Xu et al., 2014). In a systemic review and meta-analysis, the attributable fractions of HPV infection in LSCC cases were 19.1% and 8.6% according to p16 and E6/E7 mRNA expression, respectively (Ndiaye et al., 2014).
In addition, the relationship between HPV and LSCC has rarely been examined in a primary surgical cohort. Therefore, this study sought to clarify the role of HPV in LSCC and analyzed correlations among HPV, clinicopathological parameters, and clinical outcomes.
Materials and Methods
Patients and clinicopathological data
This retrospective study enrolled patients who underwent primary surgical resection with or without adjuvant radiotherapy or chemoradiotherapy between 2006 and 2009 at Chang Gung Memorial Hospital, Kaohsiung, Taiwan. Their clinicopathological characteristics were obtained from clinical records, including age, sex, T and N classification, TNM stage, tumor differentiation, histories of betel nut chewing, alcohol drinking, smoking, and survival. The TNM stage was classified according to the 2009 American Joint Committee on Cancer system as confirmed by the Head and Neck Oncology Group. This study was approved by the Medical Ethics and Human Clinical Trial Committees at Chang Gung Memorial Hospital (Ethical Application Ref: 101-3112B).
Detection of HPV
Paraffin-embedded samples from identified tumor blocks of each specimen were collected in 1.5-mL Eppendorf tubes for DNA extraction. The tumor blocks were cut after thorough cleaning of the microtome blades, and a blank paraffin section was cut as a control to prevent contamination. After the deparaffinizing procedure, genomic DNA was extracted using the QIAamp tissue kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. A total of 50 µL eluted DNA were obtained, of which 1 µL was used as the PCR template. A 192-bp HPV DNA fragment was amplified using MY11/GP6+ biotinylated consensus primers targeting the L1 region of HPV. The DNA integrity of samples was assessed by amplification of β-globin as an internal control. The pre- and post-PCR amplifications were performed in two independent rooms. The HPV-positive samples were all reconfirmed, and HPV-negative samples were randomly selected for repeat procedures to confirm the results. In the HPV-positive samples, HPV was genotyped using a commercial PCR-based reverse-blot assay (EasyChip HPV Blot; King Car, Yilan, Taiwan), which can detect 39 different HPV types. Finally, the HPV types were identified by visual assessment protocol according to the manufacturer’s instructions (Huang et al., 2006; Luo, Roan & Liu, 2007).
Statistical analysis
Fisher’s exact test was used to evaluate the correlations between clinicopathological variables and HPV status. In all statistical analyses, p-values < 0.05 and < 0.1 were considered to indicate significance and marginal significance, respectively. Variables considered in the survival analysis included age, sex, T classification, N classification, TNM stage, tumor differentiation, tumor subsite, second primary cancer, adjuvant therapy, extranodal extension, and the presence of HPV in tumor cells. The Kaplan–Meier method was used for the survival analysis, and statistical significance was defined as p < 0.05, as assessed using the log rank test.
Results
A total of 106 patients (103 men, 3 women; mean age 61.1 ± 11.8 years) were enrolled in this study. Tumor subsites included supraglottic (n = 40), glottic (n = 54), transglottic (n = 11), and subglottic (n = 1) cancers. Table 1 summarizes the clinicopathological characteristics. The incidence of a second primary cancer (synchronous or metachronous) was 17.0% (n = 18), including esophageal carcinoma in six (5.7%) patients, lung cancer in four (3.8%), other head and neck cancer subsites in six (5.7%), thyroid cancer in one (0.9%), and leukemia in one (0.9%). The second primary head and neck cancers were oral cancer in three, tonsillar cancer in two, and soft palate cancer in one patient.
| Variables | No. | HPV (−) | HPV (+) | p value |
|---|---|---|---|---|
| Gender | ||||
| Male | 103 | 89 | 14 | 1.000 |
| Female | 3 | 3 | 0 | |
| Age | ||||
| <60 y/o | 52 | 49 | 3 | 0.042* |
| ≥60 y/o | 54 | 43 | 11 | |
| T classification | ||||
| T1 and T2 | 74 | 64 | 10 | 1.000 |
| T3 and T4a | 32 | 28 | 4 | |
| N classification | ||||
| Positive | 28 | 25 | 3 | 0.756 |
| Negative | 78 | 67 | 11 | |
| TNM stage | ||||
| Stage I and II | 62 | 54 | 8 | 1.000 |
| Stage III and IV | 44 | 38 | 6 | |
| Extranodal extension | ||||
| Positive | 16 | 15 | 1 | 0.560 |
| Negative | 12 | 10 | 2 | |
| Adjuvant RT/CCRT | ||||
| Yes | 27 | 24 | 3 | 1.000 |
| No | 79 | 68 | 11 | |
| Tumor recurrence | ||||
| Yes | 23 | 23 | 0 | 0.037* |
| No | 83 | 69 | 14 | |
| Second primary cancer | ||||
| Yes | 18 | 15 | 3 | 0.703 |
| No | 88 | 77 | 11 | |
| Tumor differentiation | ||||
| Well | 28 | 25 | 3 | 0.756 |
| Moderate and poor | 78 | 67 | 11 | |
| Tumor subsites | ||||
| Transglottic | 11 | 11 | 0 | 0.117 |
| Glottic | 54 | 47 | 7 | |
| Supraglottic | 40 | 34 | 6 | |
| Subglottic | 1 | 0 | 1 | |
| Tobacco use | ||||
| Smoking | 93 | 83 | 10 | 0.068** |
| Non-smoking | 13 | 9 | 4 | |
| Alcohol use | ||||
| Drinking | 51 | 47 | 4 | 0.154 |
| Non-drinking | 55 | 45 | 10 | |
| Betel nut chewing | ||||
| Chewing | 53 | 46 | 7 | 1.000 |
| Non-chewing | 53 | 46 | 7 |
HPV genotyping
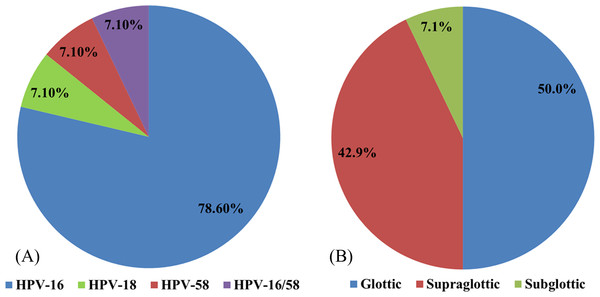
HPV was detected in 14 patients (13.2%): 11 (78.6%) specimens were positive for HPV-16, one (7.1%) for HPV-18, one (7.1%) for HPV-58, and one (7.1%) for both HPV-16 and -58 (Fig. 1A). HPV was present in the glottic, supraglottic, and subglottic laryngeal subsites in seven (50%), six (42.9%), and one (7.1%) patients, respectively (Fig. 1B).
Figure 1: The distributions of different HPV types.
The distributions of (A) various HPV types in laryngeal cancer and (B) HPV at different laryngeal subsites.Clinicopathological parameters
The patients with HPV-positive tumors were significantly older (p = 0.042) and had a higher local/regional control rate (p = 0.037) than that of patients with HPV-negative tumors. Fewer patients with HPV-positive tumors were smokers, although this had marginal significance (p = 0.068; Table 1).
| Variable | No | OS | p-value | DSS | p-value | Recurrence- free | p-value |
|---|---|---|---|---|---|---|---|
| HPV status | |||||||
| Positive | 14 | 64.3 | 0.8056 | 100 | 0.1485 | 100 | 0.0494* |
| Negative | 92 | 67.4 | 84.8 | 75.0 | |||
| Gender | |||||||
| Male | 103 | 67.0 | 0.9186 | 87.4 | 0.3010 | 79.6 | 0.0490* |
| Female | 3 | 66.7 | 66.7 | 33.3 | |||
| Age | |||||||
| <60 y/o | 52 | 76.9 | 0.0416* | 86.5 | 0.9345 | 78.9 | 0.8140 |
| ≥60 y/o | 54 | 57.4 | 87.0 | 77.8 | |||
| Tumor differentiation | |||||||
| Well | 28 | 78.6 | 0.1416 | 96.4 | 0.0818 | 78.6 | 0.9210 |
| Moderate/poor | 78 | 62.8 | 83.3 | 78.2 | |||
| Tumor site | |||||||
| Glottic | 54 | 81.5 | 0.0011* | 96.3 | 0.0021* | 81.5 | 0.2499 |
| Non-Glottic | 52 | 51.9 | 76.9 | 75.0 | |||
| ENE | |||||||
| Yes | 16 | 37.5 | 0.1563 | 56.3 | 0.1177 | 68.8 | 0.1230 |
| No | 12 | 66.7 | 83.3 | 91.7 | |||
| T classification | |||||||
| T1 and T2 | 74 | 70.3 | 0.1762 | 90.5 | 0.0589** | 79.7 | 0.4137 |
| T3 and T4a | 32 | 59.4 | 78.1 | 75.0 | |||
| N classification | |||||||
| Negative | 78 | 73.1 | 0.0161* | 93.6 | 0.0003* | 78.2 | 0.9535 |
| Positive | 28 | 50.0 | 67.9 | 78.6 | |||
| TNM stage | |||||||
| I, II | 62 | 72.6 | 0.1044 | 95.2 | 0.0021* | 80.7 | 0.3744 |
| III, IVa | 44 | 59.1 | 75.0 | 75.0 | |||
| Second primary cancer | |||||||
| Yes | 18 | 44.4 | 0.0338* | 100 | 0.1059 | 88.9 | 0.3066 |
| No | 88 | 71.6 | 84.1 | 76.1 | |||
| Adjuvant RT/CCRT | |||||||
| Yes | 27 | 63.0 | 0.5087 | 81.5 | 88.9 | 0.1271 | |
| No | 79 | 68.4 | 88.6 | 0.3121 | 74.7 |
Survival analysis
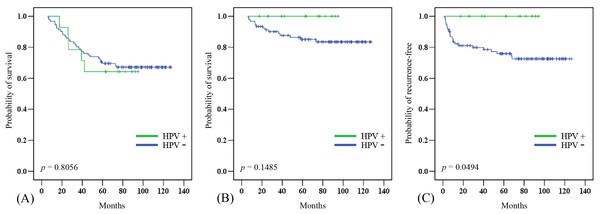
In this cohort, the 5-year overall (OS) and disease-specific (DSS) survival rates were 67.0% and 86.8%, respectively. The median follow-up period was 82.7 (range 6.0–127.4) months. Twenty-one patients died of diseases other than laryngeal cancer, in most cases chronic obstructive pulmonary disease (COPD), lung cancer, or esophageal cancer. The 5-year OS was significantly poorer in patients who were older (p = 0.0416), had a positive N classification (p = 0.0161), had a second primary cancer (p = 0.0338), and had non-glottic cancer (p = 0.0011). The 5-year DSS was significantly poorer in patients with a positive N classification (p = 0.0003), advanced TNM stage (p = 0.0021), and non-glottic cancer (p = 0.0021) (Table 2). There was no significant difference in the 5-year OS between HPV-positive and -negative tumors (Fig. 2A). The patients with HPV-positive tumors had a better 5-year DSS (100% vs. 84.8%, p = 0.1485), although the difference did not reach statistical significance (Fig. 2B). However, the 5-year local/regional control rate was significantly better in HPV- positive tumors than in HPV-negative tumors (100% vs. 75%, p = 0.0494; Fig. 2C).
Figure 2: Clinical outcome in HPV-positive and HPV-negative tumors.
The effect of HPV status on (A) overall survival, (B) disease-specific survival, and (C) local/regional control rates.Discussion
Smoking and drinking are the main risk factors for head and neck cancer. Recently, HPV has been shown to be a new pivotal factor in the development of HNSCC, specifically in OPSCC (Stenmark et al., 2017; Wang et al., 2017). However, the role of HPV in head and neck cancers other than OPSCC remains unclear. In oral squamous cell carcinoma (OSCC), although Zafereo et al. (2016) found a high incidence of p16 overexpression (especially in the oral tongue area, 36.3%), only 6% of OSCC cases were considered HPV-driven tumors. In LSCC, the exact role of HPV infection remains controversial, and the prevalence varies widely from 6.8% to 58.8%, with an average prevalence of 28% reported in a recent meta-analysis (Ma et al., 1998; Fouret et al., 1997; Li et al., 2013).
Several factors may contribute to the high variation in HPV prevalence. Lindeberg & Krogdahl (1999) suggested that a higher prevalence could be explained by a high frequency of false-positive results caused by sample contamination. With the technical advances in HPV detection, the prevalence of HPV in patients with LSCC has been quite low in recent publications. In two recent studies involving a large patient series from China and the UK, the prevalence was only 7.57% and 3.2%, respectively (Upile et al., 2014; Xu et al., 2014). Another international cross-sectional study of 3,680 head and neck cancer samples found a low HPV prevalence, except in patients with OPSCC, and only 3.5% of laryngeal cancers were HPV-positive (Castellsagué et al., 2016). We also found a relatively low HPV prevalence: 13.2% of patients with LSCC in Taiwan.
Geographic differences may also contribute to the wide range of HPV infection rates in LSCC. Unlike other countries, betel quid chewing is an important threat to public health in Taiwan in addition to smoking and drinking. Patients who habitually chew betel nut were found to have a higher incidence of supraglottic cancer versus glottic cancer (52.8% vs. 35.8%, p = 0.003). This suggested that betel nut chewing is a risk factor for the development of supraglottic cancer, in addition to smoking and drinking.
In our study, HPV-16 was the major HPV type, as in other reports (Kreimer et al., 2005). No low-risk HPV types were detected in patients with LSCC in our cohort. Unlike the epidemiological signature of patients with HPV-positive OPSCC, younger patients and those with early T stage disease with extensive nodal metastasis showed no consistent characteristic findings in HPV-positive LSCC (Mallen-St Clair et al., 2016). Xu et al. (2014) found that HPV-positive tumors were associated with supraglottic cancer, non-smokers, and non-drinkers. Hernandez et al. (2014) found a higher prevalence of HPV-positive tumors in women and in patients with node-positive cancer or metastasis. Gillison et al. (2000) found that HPV-positive tumors were more likely to be poorly differentiated. However, we did not find any association of HPV status with clinical stage, nodal metastasis, secondary aerodigestive cancer, tumor subsites, or tumor differentiation (Table 1). In our series, patients with HPV-positive tumors were significantly older and marginally significantly non-smokers (p = 0.068). This result is compatible with those of Xu et al. (2014) and Baumann et al. (2009).
Only a few studies have reported the prognosis of HPV-positive LSCC, and these studies failed to show an improved OS (Xu et al., 2014; Hernandez et al., 2014; Shaughnessy et al., 2014). In our series, the OS may not actually reflect the survival advantage of HPV-positive LSCC tumors, since only 14 of 35 deaths were attributed to LSCC. One-third of the deaths were caused by lung cancer, COPD, or esophageal cancer. In addition, none of the patients with HPV-positive LSCC experienced treatment failure, showing 100% local/regional control. HPV-positive LSCC showed a trend toward a better 5-year DSS (100% vs. 84.8%, p = 0.1485) and a significant improvement in the local/regional control rate (100% vs. 75%, p = 0.0494). Less aggressive tumor behavior and a better response to adjuvant radiotherapy/concurrent chemoradiotherapy of HPV-positive tumors were possible causes of these clinical outcomes. Shaughnessy et al. (2014) observed an improvement in 2-year DFS in HPV-positive laryngeal and hypopharyngeal cancer patients treated with chemoradiotherapy, but they did not specify the results for LSCC. Although previous reports have not observed a survival advantage in HPV-positive LSCC, we found that patients with HPV-positive LSCC had 100% 5-DSS and 100% local/regional control rates if they underwent primary surgery.
To the best of our knowledge, this is the first report on HPV prevalence in LSCC in Taiwan, where habitual betel nut chewing is common. A limitation of this study is that the presence of HPV DNA in LSCC does not provide sufficient evidence for HPV-induced carcinogenesis. Further biomarkers including p16 and E6/E7 mRNA should be investigated to determine oncogenic activity. However, p16 overexpression is not as reliable of a marker for LSCC as for OPSCC, since it may be triggered by other pathways (Ndiaye et al., 2014). For determination of HPV-induced LSCC in clinical settings, Fusconi et al. (2017) proposed that detection of HPV DNA is the first step, followed by detection of E6/E7 mRNA in positive cases. Currently, limited data on HPV mRNA in LSCC are available, and further prospective studies are required to clarify its roles in prognosis and therapeutic efficacy.
Conclusions
The prevalence of HPV in patients with LSCC was only 13.2% in our series. The low prevalence of HPV infection suggests that HPV is not a major cause of LSCC. In addition to smoking and drinking, betel nut chewing increases the risk of supraglottic cancer. HPV-related tumors had no significant impact on OS, although a 100% local/regional control rate and 100% 5-year DSS were observed in the patients who underwent primary surgery to treat LSCC. However, a larger sample size is necessary to confirm our observations.