Abstract
Aims: Patients with diabetes mellitus have a higher risk of adverse events after percutaneous coronary intervention (PCI). This study aimed to elucidate the relative efficacy of everolimus-eluting stents (EES) versus sirolimus-eluting stents (SES) according to diabetic status.
Methods and results: Data from the EXCELLENT randomised trial and registry were pooled in a per protocol analysis manner. The primary endpoint was target lesion failure (TLF), a composite of cardiac death, target vessel-related myocardial infarction, or target vessel revascularisation. Among a total of 6,524 patients, 2,404 (36.8%) had diabetes mellitus. Patients with diabetes were shown to have a higher rate of TLF after PCI, which was mainly driven by differences in cardiac death and myocardial infarction, while the rate of repeat revascularisation and stent thrombosis did not differ significantly. TLF occurred at a similar rate between patients treated with EES versus SES in each subgroup stratified by diabetic status (interaction p=0.384). In addition, no significant interactions were present with regard to any pre-specified clinical endpoints. The results were corroborated by analysis with inverse probability of treatment weighting (interaction p=0.329). We also found that insulin-dependent diabetes imposed an even greater risk of TLF on patients treated with PCI.
Conclusions: Despite the recent advances in drug-eluting stent technology, diabetic patients are still at higher risk of adverse clinical events after PCI than those without diabetes mellitus. Whether a patient was treated with EES or SES had no significant interaction with diabetic status in terms of clinical outcome after PCI.
Introduction
Patients with diabetes mellitus are prone to a higher incidence of adverse clinical events as well as angiographic restenosis after percutaneous coronary intervention (PCI)1,2. Despite the development of drug-eluting stents (DES), which have been shown to reduce the need for repeat revascularisation compared to bare metal stents in patients with diabetes mellitus3-5, diabetes still remains one of the major predictors for poor clinical outcomes6-8. The selection of optimal DES providing the best performance is one of the main issues in the treatment of diabetic patients with coronary artery disease.
Previous studies have proven an interaction between the performance of different types of DES and diabetic status. Everolimus-eluting stents (EES), when compared to paclitaxel-eluting stents, were shown to reduce the risk of adverse outcomes among patients without diabetes mellitus significantly. Meanwhile, diabetic patients showed no significant differences in terms of safety and efficacy outcomes when treated with EES or paclitaxel-eluting stents9,10. Although controversial, studies comparing sirolimus-eluting stents (SES) versus paclitaxel-eluting stents also suggested the attenuation of antirestenotic effects of SES in diabetic subgroups11-13. So far, the question as to whether different types of limus-eluting stents, namely EES compared to SES, have relative efficacy according to diabetic status in terms of clinical outcomes after PCI has not been fully investigated. To address this issue, we compared one-year clinical outcomes after implantation of EES versus SES in patients with and without diabetes mellitus.
Methods
PATIENTS
For the purpose of this study, we pooled the database from the EXCELLENT randomised trial and the non-randomised all-comers EXCELLENT registry with the agreement of both steering committees. The Efficacy of Xience/promus versus Cypher in rEducing Late Loss after stENTing (EXCELLENT) trial was a prospective, randomised, multicentre trial which enrolled 1,443 patients between June 2008 and July 2009 in order to compare the efficacy of EES versus SES in reducing late loss in patients undergoing PCI. The study design and the primary results have been reported previously14-16. The EXCELLENT registry was an open-label, multicentre, all-comers registry where consecutive patients receiving EES were prospectively enrolled between May 2008 and May 2010 while a historical control of consecutive patients who received SES between January 2004 and April 2009 was retrospectively registered from 29 centres in Korea. The patients enrolled in the EXCELLENT registry were completely distinct from those enrolled in the EXCELLENT randomised trial17. Patients were considered as having diabetes mellitus if they had a history of diabetes diagnosed and/or treated by a healthcare provider. Diabetic status was classified as insulin-dependent if the patient was taking insulin, or non-insulin-dependent if he/she was taking only oral hypoglycaemic agents or treated with diet therapy.
The study protocols were approved by the ethics committee at each participating centre, and followed the principles of the Declaration of Helsinki. All patients provided written, informed consent for participation in the trial. Patients were grouped into the EES and SES groups on a per protocol basis. The study scheme is summarised in Figure 1.
STUDY ENDPOINTS
The primary analysis endpoint of the present study was target lesion failure (TLF), a composite of cardiac death, target vessel-related myocardial infarction (MI), and clinically-driven target lesion revascularisation (TLR) at one year. Secondary endpoints included all-cause death, cardiac death, all-cause MI, target vessel-related MI, target vessel revascularisation (TVR), TLR, and stent thrombosis. All definitions of clinical events followed the consensus of the Academic Research Consortium (ARC)18. Specifically, cardiac death was defined as any death due to a cardiac cause, unwitnessed death and death of unknown cause, and all procedure-related deaths. MI included those occurring during the immediate periprocedural period (within 48 hours after PCI) and longer after the procedure (more than 48 hours after PCI). Whether MI was related to target vessel or not was adjudicated on the basis of angiographic and/or electrocardiographic data. TLR was defined as any repeat percutaneous or surgical intervention of the target lesion, which means the treated segment from 5 mm proximal to the stent and up to 5 mm distal to the stent. The occurrence of “definite” and “probable” stent thrombosis (ST), according to ARC definition, was recorded. Target vessel failure was defined as a composite of cardiac death, target vessel-related MI, and clinically-driven TLR, while a major adverse cardiovascular event was as a composite of all-cause death, all-cause MI, TVR, and ST. Clinical events were adjudicated by an independent adjudication committee in both the EXCELLENT trial and the EXCELLENT registry.
STATISTICAL ANALYSIS
Clinical outcomes were compared between patients treated with EES versus SES stratified by the presence of diabetes mellitus. Baseline data were presented as frequencies or mean±SD. Categorical variables were compared with the use of the χ2 test or Fisher’s exact test, and continuous variables with the Student’s t-test. The event-free survival rates were analysed by the Kaplan-Meier method, and the differences in survival curves between the groups were assessed with the log-rank test. Interaction was tested with use of the Cox proportional hazards model to determine whether the presence of diabetes mellitus had an effect on the relative risk of EES versus SES regarding any clinical endpoints.
In order to minimise selection bias in this study, weighted Cox proportional hazards regression models using the inverse probability of treatment weights (IPTW) was used19. Propensity to the treatment with EES compared to SES was scored by the use of multivariable logistic regression for every patient with 24 clinical and angiographic variables, such as sex, age, body mass index, hypertension, dyslipidaemia, smoking status, the presence of peripheral artery disease, family history of coronary artery disease, clinical diagnosis, previous PCI, previous bypass surgery, history of myocardial infarction, congestive heart failure, chronic renal failure, ejection fraction estimated with echocardiography, extent of diseased coronary artery, stent diameter, total stent length, number of lesions, number of implanted stents, left main coronary artery disease, bifurcation lesion, and the presence of thrombus. A multiple imputation method was used to fill out missing variables with the assumption that data were missing completely at random. No interactions were considered in the model. Propensity scores were used to derive the IPTW, with the inverse of the propensity score for patients treated with EES and the inverse of (1 - propensity score) for patients with SES. Clinical outcomes were compared with the use of logistic regression models adjusted with the inverse probability of treatment weighting. A two-sided p-value less than 0.05 was considered significant for all tests. All statistical analyses were performed using IBM SPSS version 18.0 (IBM Corp., Armonk, NY, USA).
Results
PATIENT CHARACTERISTICS
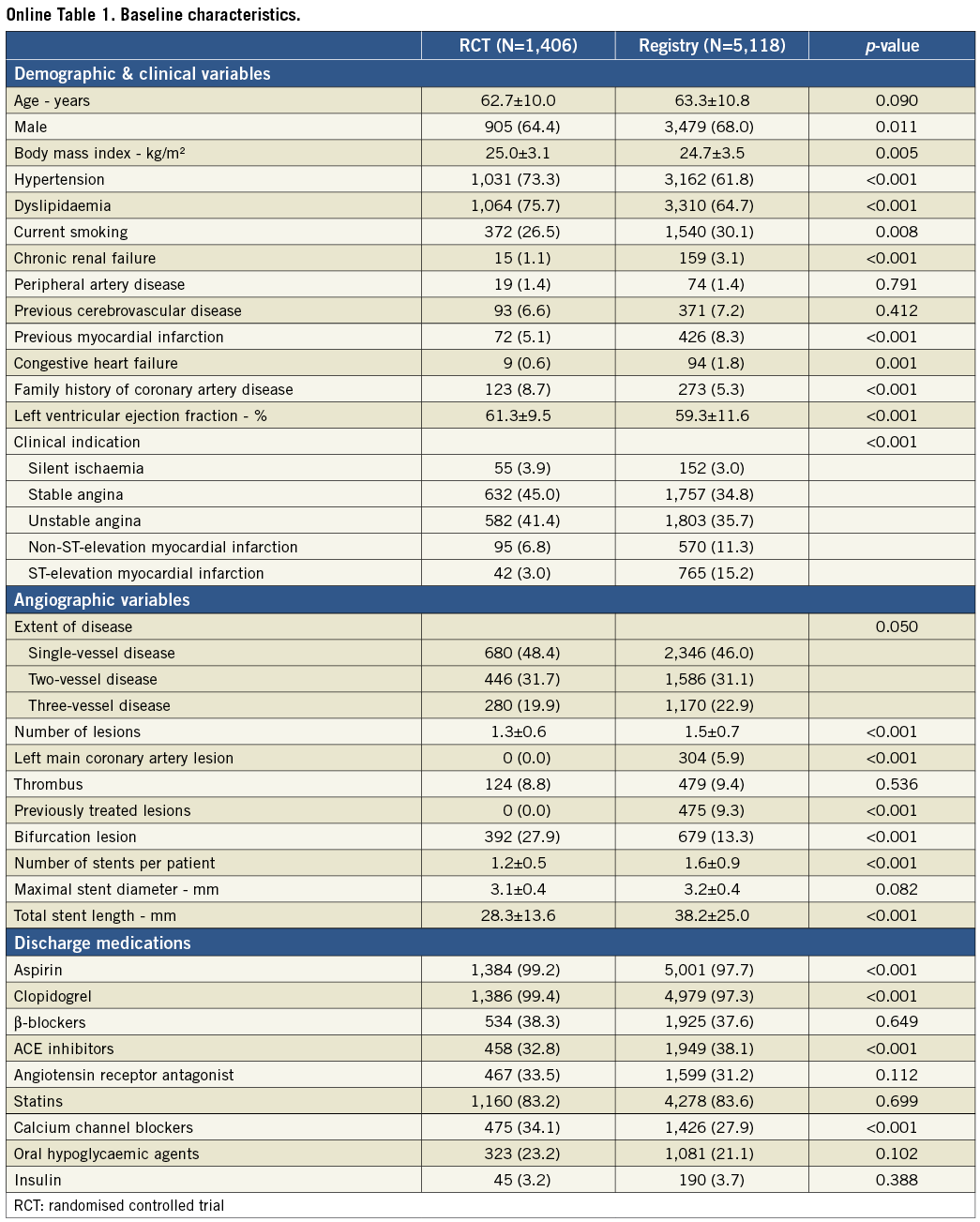
The study and analysis scheme is shown in Figure 1. As shown in Online Table 1, patients from the EXCELLENT registry represented a higher risk profile, in terms of both clinical and angiographic factors, than those enrolled in the randomised trial. Among a total of 6,524 study patients, 2,404 (36.8%) had diabetes mellitus, while the other 4,120 (63.2%) did not. Table 1 compares the baseline characteristics of patients treated with EES versus SES according to diabetic status. In terms of demographic variables, patients in the EES group had higher frequencies of hypertension and dyslipidaemia, and a lower frequency of ST-segment elevation myocardial infarction regardless of diabetic status. In patients without diabetes, the EES group had a higher mean age, a lower frequency of previous MI, and a higher frequency of congestive heart failure. Regarding angiographic characteristics, the proportion of previously treated lesions was lower, maximal stent diameter was larger, and total stent length was shorter in the EES group compared to the SES group. Discharge medications were mostly comparable between the two groups.
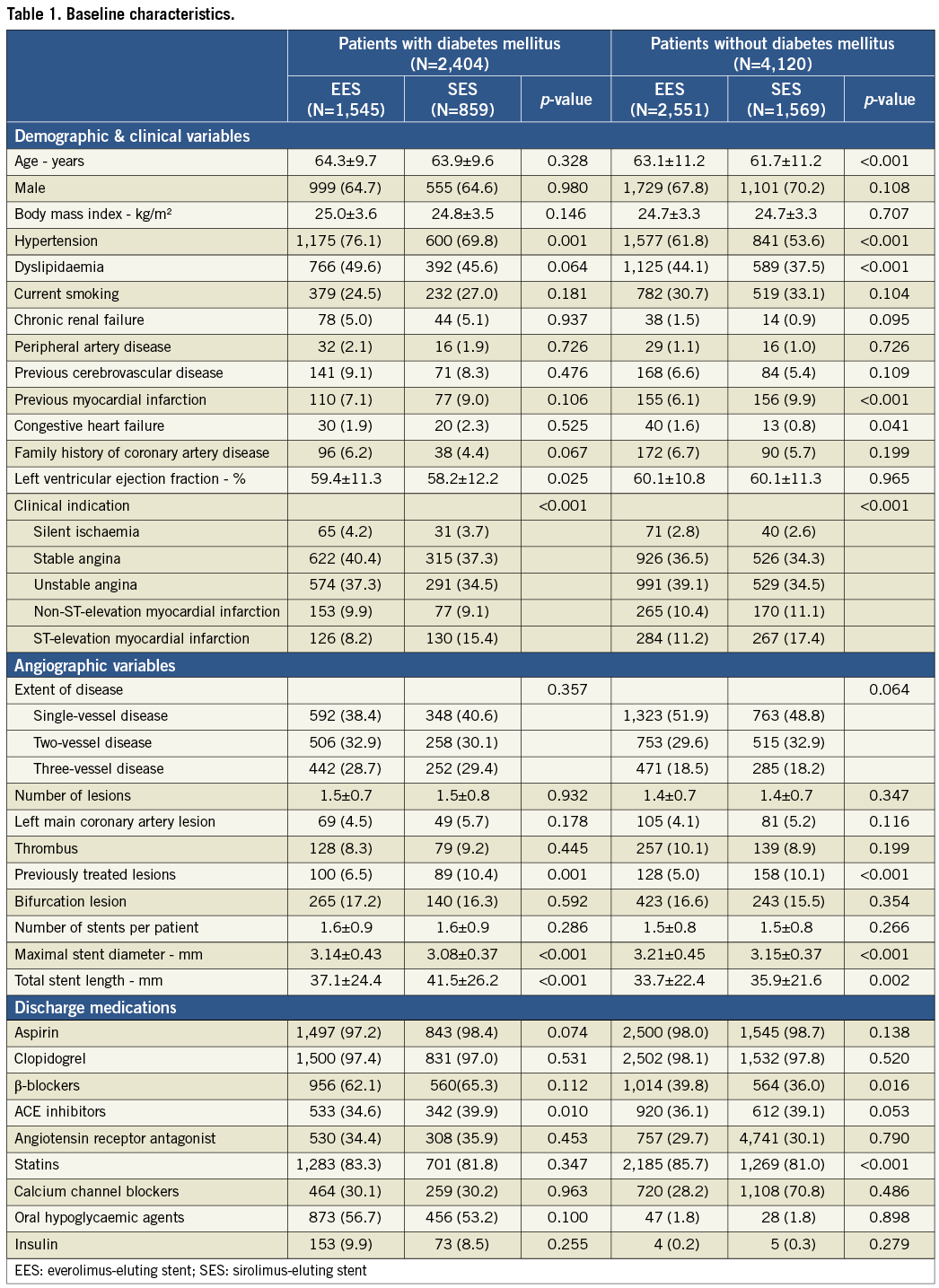
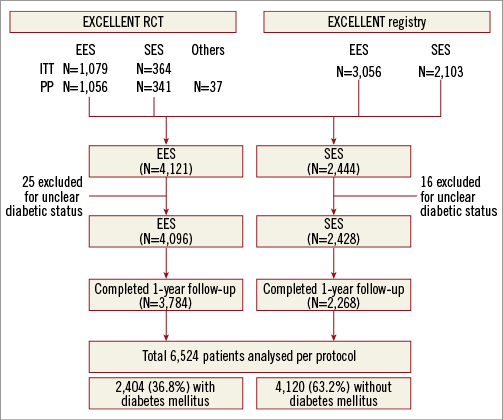
Figure 1. Study protocol. EES: everolimus-eluting stent; ITT: intention-to-treat; PP: per protocol; RCT: randomised controlled trial; SES: sirolimus-eluting stent
CLINICAL OUTCOMES
Compared with non-diabetics, those with diabetes mellitus showed poorer clinical outcomes with respect to all composite and individual outcome variables (Figure 2). All pre-specified clinical endpoints, including the primary endpoint, occurred at significantly higher rates in diabetics, except for TVR, TLR, and ST, which were all numerically higher in diabetic patients.
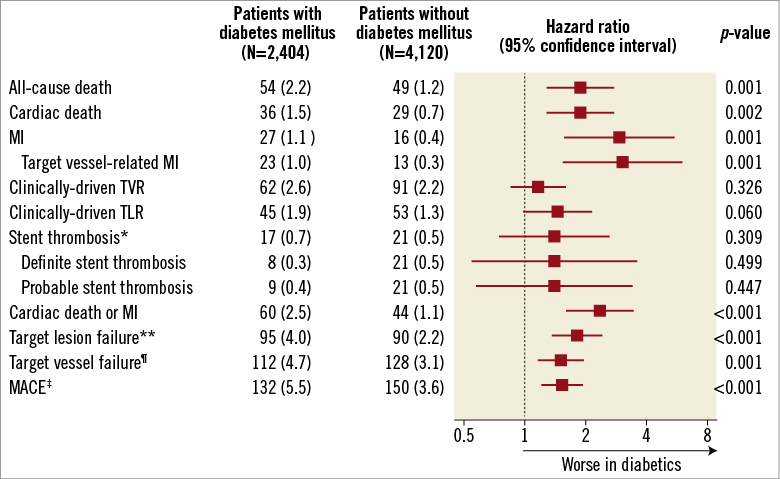
Figure 2. Clinical outcomes at one year in patients with and without diabetes mellitus. *Stent thrombosis was defined according to the consensus of the Academic Research Consortium. **Target lesion failure was a composite of cardiac death, target vessel-related MI, or clinically-driven TLR. ¶Target vessel failure was a composite of cardiac death, target vessel-related MI, or clinically-driven TVR. ‡MACE was a composite of all-cause death, all-cause MI, or clinically-driven TVR. MACE: major adverse cardiac events; MI: myocardial infarction; TLR: target lesion revascularisation; TVR: target vessel revascularisation
Figure 3 shows the Kaplan-Meier survival curves comparing the incidence of the primary endpoint between EES and SES according to the presence (Figure 3A) or absence of diabetes mellitus (Figure 3B). While the absolute rate of TLF was higher in patients with diabetes, both stents showed similar outcomes when compared head-to-head in each subgroup of diabetics and non-diabetics. The interaction between the type of stent and diabetes was not significant (p=0.384). As shown in Table 2, there were no significant interactions for any of the secondary endpoints.
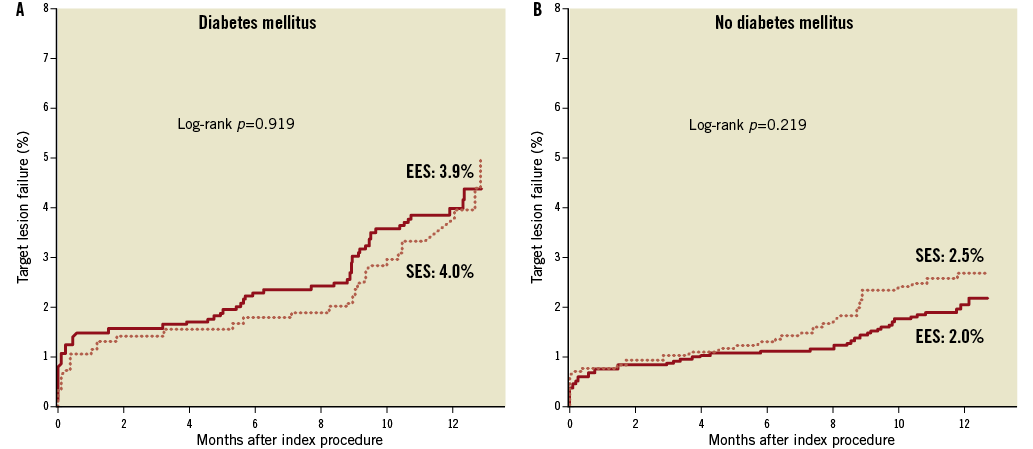
Figure 3. Kaplan-Meier survival curves of target lesion failure in patients treated with everolimus-eluting stents (EES) versus sirolimus-eluting stents (SES) according to diabetic status. EES: everolimus-eluting stent; SES: sirolimus-eluting stent
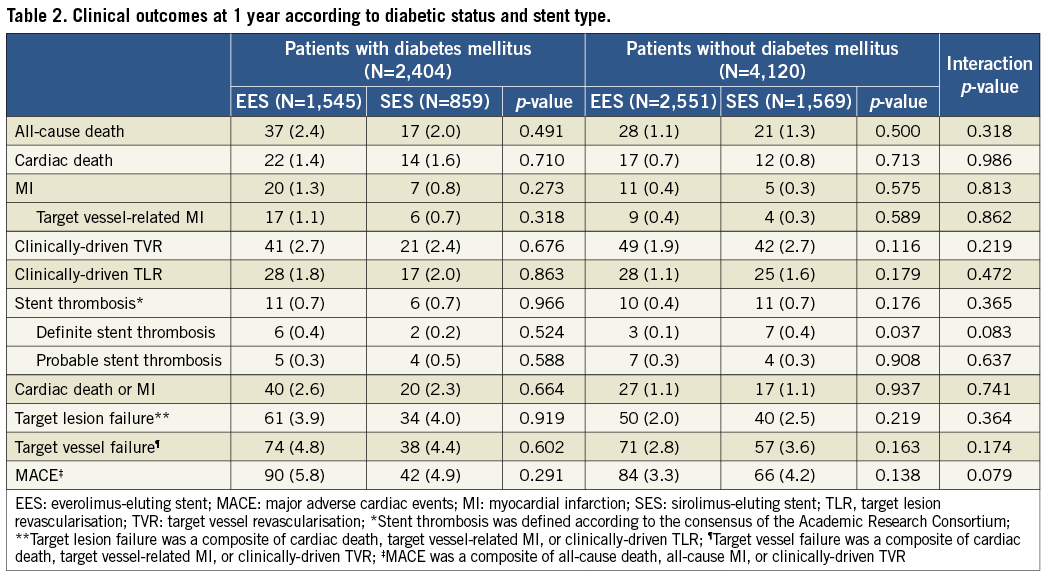
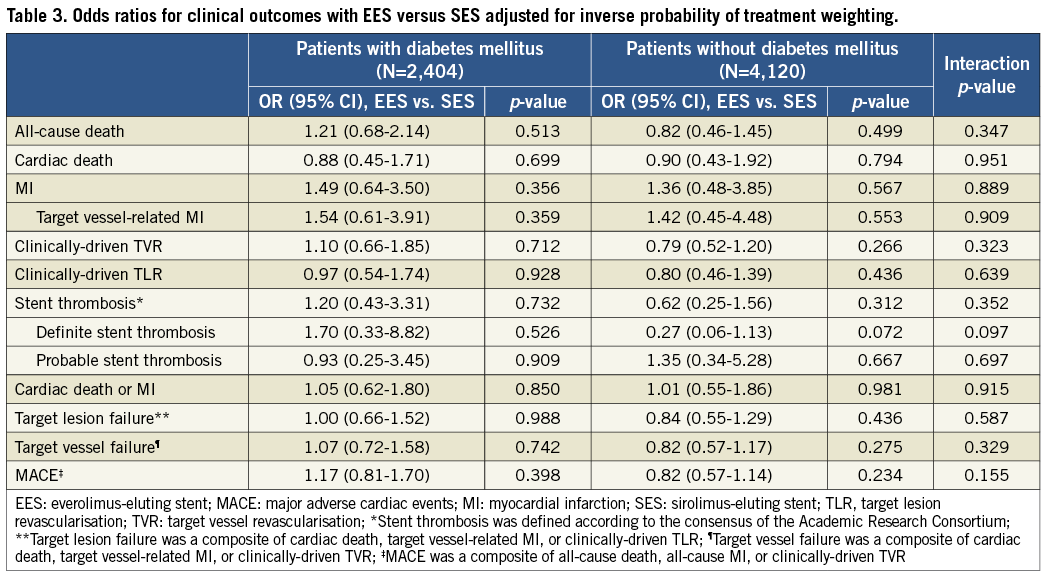
Adjusted analyses with IPTW confirmed the results from the crude population (Table 3). There were no significant differences between EES and SES in any of the pre-specified endpoints regardless of diabetic status, while no interaction was found to be significant.
IMPACT OF DIABETES MELLITUS SEVERITY
Among diabetics, 184 (11.9%) and 90 (10.5%) patients had insulin-dependent diabetes in the EES and SES groups, respectively. We found a linear relationship between the severity of diabetes and the occurrence of clinical events (Figure 4). Although p-values were insignificant for ST, and TLR in the SES group, there was a tendency for numerical increment. No interaction between stent type and diabetic severity was present regarding any measured parameters.
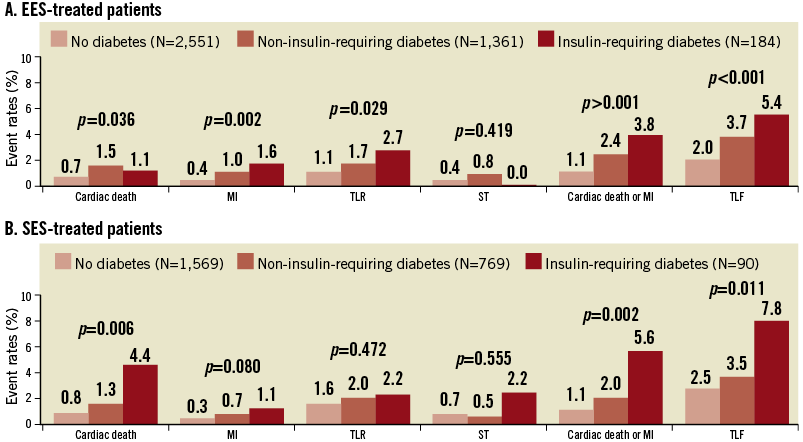
Figure 4. Event rates in patients without diabetes, with diabetes not requiring insulin, and with diabetes requiring insulin. MI: myocardial infarction; ST: stent thrombosis; TLF: target lesion failure; TLR: target lesion revascularisation; TVR: target vessel revascularisation
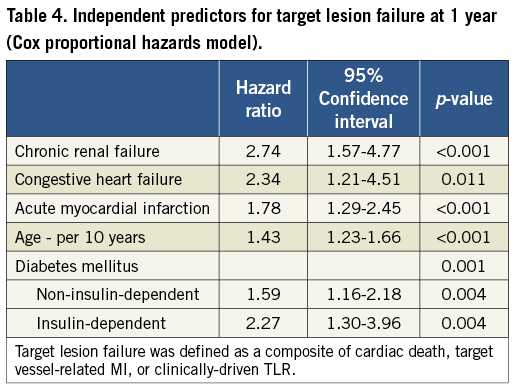
The Cox regression model with a forward stepwise method was used to find the independent predictors for TLF. The major contributing factors for TLF were shown to be age, congestive heart failure, chronic renal failure, and clinical diagnosis of acute myocardial infarction, as well as diabetes with a gradient according to its severity. The hazard ratio with the reference of the absence of diabetes was 1.59 (p=0.004) for non-insulin-dependent diabetes, and 2.27 (p=0.004) for insulin-dependent diabetes (Table 4).
Discussion
The major finding of this study is that, in a large PCI population with DES, diabetes mellitus is still a major predictor of adverse events. In addition, the impact of diabetes was consistent regardless of the type of “limus” stent implanted. Moreover, the increase in TLF in diabetic patients was driven not only by repeat revascularisation, but also by hard endpoints including cardiac death and MI. Finally, insulin-dependent diabetes imposed an even higher risk of adverse events than non-insulin-dependent diabetes.
The selection of the optimal treatment strategy for diabetic patients, including medical, interventional, and surgical therapy, has been an important issue among cardiovascular physicians. Diabetes is associated with a twofold to fourfold increase in the risk of developing coronary artery disease20, and patients with diabetes usually have worse outcomes after PCI1,2. Although the introduction of DES has potentially reduced the need for repeat revascularisation, diabetic status is still associated with an increased risk for adverse cardiac events in patients undergoing PCI6-8. Interestingly, previous studies have demonstrated an interaction between stent type and diabetes in terms of clinical outcomes after PCI: while limus-eluting stents (i.e., EES or SES) show worse outcomes when implanted to diabetic patients, the performance of paclitaxel-eluting stents remains similar9-13. This phenomenon is explained by the different mechanisms of action through which paclitaxel and rapamycin analogues reduce restenosis: whereas paclitaxel interferes with multiple pathways of restenosis, rapamycin analogues work via blocking the PI3K/AKT/mTOR signal axis, a pathway which is already weakened in type II diabetes21,22.
To the best of our knowledge, this is the largest study to assess the interaction between diabetic status and the use of the two “limus-eluting” stents, EES and SES, which are currently the most widely used among DES. Kim et al showed in the ESSENCE-DIABETES randomised trial that, among diabetic patients, EES was non-inferior to SES in terms of angiographic outcomes23. A substudy of the SORT OUT IV trial showed no significant differences in clinical outcomes between EES and SES in diabetic or non-diabetic patients24. Kufner et al also showed comparable outcomes between EES and SES in patients with diabetes enrolled in the ISAR-TEST-4 trial25. Our study has the merit that it reflects a broader study population, as we pooled patients from a randomised trial with strict inclusion criteria and a “real-world” robust registry. These results dispel those concerns that the issue of attenuated efficacy may be a problem specific to EES.
It is notable that in this study the risk of device-specific clinical events (i.e., TLR, TVR, and ST) did not differ significantly between diabetics and non-diabetics. Similarly, a recent pooled analysis of RESOLUTE programmes also showed no significant differences between diabetics and non-diabetics as well as insulin-treated and non-insulin-taking patients26. These results should be interpreted with caution, because the occurrence of those events was still numerically high. Although a statistical significance has disappeared due to the low statistical power provoked by the improved performance of recently developed DES, the relative risk still remains the same: risk ratio of TLR and TVR between 1.2-1.5, and that of ST around 1.510,26.
Despite recent advances in interventional devices, this study showed that diabetes mellitus still remains a hurdle to be overcome when a patient with coronary artery disease is treated with PCI. Further improvement in device technology may be able to tackle this issue. However, one of the major findings of this study is that the increase in the primary endpoint originated more from cardiac death and MI rather than from device-specific endpoints. Thus, it can be speculated that there is little room for device innovation. The remaining question may be whether more intensive glycaemic control would improve outcomes after PCI in diabetic patients. This needs to be investigated in future trials.
Limitations
This study has several limitations. First, as we pooled data from a randomised trial and an observational registry, the analysed cohort was heterogeneous. Although this is the largest study testing the relative efficacy of EES versus SES stratified by the status of diabetes mellitus, the results should be considered hypothesis-generating at best. Second, clinical follow-up was restricted to one year. Late or very late stent thrombosis is the major concern regarding the safety of DES, and this could not be captured in this study. Third, patients with diabetes mellitus show a high frequency of silent myocardial ischaemia. As this study did not mandate angiographic follow-up, recurrent ischaemia could have been overlooked. Fourth, as event rates were low in this study, we cannot exclude that the study was underpowered.
Conclusions
In this study, we found that patients with diabetes mellitus were at increased risk of adverse cardiac events after PCI when treated with EES or SES. Furthermore, the increased risk in diabetics compared to non-diabetics had no significant interaction with whether the patient was treated with EES or SES.
Acknowledgements
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A102065), and a grant from the Innovative Research Institute for Cell Therapy, Seoul National University Hospital (A062260), sponsored by the Ministry of Health and Welfare, Republic of Korea.
Conflict of interest statement
The authors have no conflicts of interest to declare.