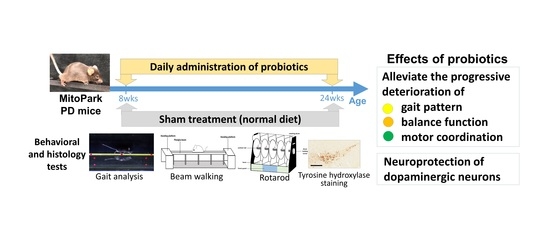
Probiotics Alleviate the Progressive Deterioration of Motor Functions in a Mouse Model of Parkinson’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Probiotics
2.3. Behavioral Tests
2.3.1. Beam Balance Test
2.3.2. Rotarod Testing
2.3.3. Gait Analysis
2.4. Histology Investigation
2.5. Statistical Analysis
3. Results
3.1. Long-Term Probiotic Treatment Mitigates Beam Balance and Motor Coordination in MitoPark PD Mouse Model
3.2. Effect of Probiotics on Gait Pattern
3.3. Effect of Probiotic Treatment Tested by Immunohistochemistry (IHC) in PD Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiang, P.; Dickson, D.W. Parkinson’s disease: Experimental models and reality. Acta Neuropathol. 2018, 135, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Rial, D.; Castro, A.A.; Machado, N.; Garcao, P.; Goncalves, F.Q.; Silva, H.B.; Tome, A.R.; Kofalvi, A.; Corti, O.; Raisman-Vozari, R.; et al. Behavioral phenotyping of Parkin-deficient mice: Looking for early preclinical features of Parkinson’s disease. PLoS ONE 2014, 9, e114216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirier, A.A.; Aube, B.; Cote, M.; Morin, N.; Di Paolo, T.; Soulet, D. Gastrointestinal Dysfunctions in Parkinson’s Disease: Symptoms and Treatments. Parkinsons Dis. 2016, 2016, 6762528. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, M.G.; Benarroch, E.E. Autonomic involvement in Parkinson’s disease: Pathology, pathophysiology, clinical features and possible peripheral biomarkers. J. Neurol. Sci. 2012, 313, 57–63. [Google Scholar] [CrossRef]
- Woitalla, D.; Kassubek, J.; Timmermann, L.; Lauterbach, T.; Berkels, R.; Grieger, F.; Muller, T. Reduction of gastrointestinal symptoms in Parkinson’s disease after a switch from oral therapy to rotigotine transdermal patch: A non-interventional prospective multicenter trial. Parkinsonism Relat. Disord. 2015, 21, 199–204. [Google Scholar] [CrossRef]
- Salari, M.; Fayyazi, E.; Mirmosayyeb, O. Gastrointestinal dysfunction in idiopathic Parkinsonism: A narrative review. J. Res. Med. Sci. 2016, 21, 126. [Google Scholar] [CrossRef]
- Leopold, N.A.; Kagel, M.C. Prepharyngeal dysphagia in Parkinson’s disease. Dysphagia 1996, 11, 14–22. [Google Scholar] [CrossRef]
- Dembinski, A.; Warzecha, Z.; Ceranowicz, P.; Dembinski, M.; Cieszkowski, J.; Gosiewski, T.; Bulanda, M.; Kusnierz-Cabala, B.; Galazka, K.; Konturek, P.C. Synergic Interaction of Rifaximin and Mutaflor (Escherichia coli Nissle 1917) in the Treatment of Acetic Acid-Induced Colitis in Rats. Gastroenterol. Res. Pract. 2016, 2016, 3126280. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.D.; Rahmani, E.; Garcia, E.; Jacobs, J.P. Gastrointestinal symptoms are predictive of trajectories of cognitive functioning in de novo Parkinson’s disease. Parkinsonism Relat. Disord. 2020, 72, 7–12. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.; Jin, F. Gut-Brain Psychology: Rethinking Psychology From the Microbiota-Gut-Brain Axis. Front. Integr. Neurosci. 2018, 12, 33. [Google Scholar] [CrossRef]
- Chapelet, G.; Leclair-Visonneau, L.; Clairembault, T.; Neunlist, M.; Derkinderen, P. Can the gut be the missing piece in uncovering PD pathogenesis? Parkinsonism Relat. Disord. 2019, 59, 26–31. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caputi, V.; Giron, M.C. Microbiome-Gut-Brain Axis and Toll-Like Receptors in Parkinson’s Disease. Int. J. Mol. Sci. 2018, 19, 1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pendharkar, S.A.; Asrani, V.M.; Murphy, R.; Cutfield, R.; Windsor, J.A.; Petrov, M.S. The Role of Gut-brain Axis in Regulating Glucose Metabolism After Acute Pancreatitis. Clin. Transl. Gastroenterol. 2017, 8, e210. [Google Scholar] [CrossRef] [PubMed]
- Borre, Y.E.; O’Keeffe, G.W.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends Mol. Med. 2014, 20, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Mulak, A.; Bonaz, B. Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroenterol. 2015, 21, 10609–10620. [Google Scholar] [CrossRef] [PubMed]
- Konturek, S.J.; Pepera, J.; Zabielski, K.; Konturek, P.C.; Pawlik, T.; Szlachcic, A.; Hahn, E.G. Brain-gut axis in pancreatic secretion and appetite control. J. Physiol. Pharmacol. 2003, 54, 293–317. [Google Scholar]
- Nirmalkar, K.; Murugesan, S.; Pizano-Zarate, M.L.; Villalobos-Flores, L.E.; Garcia-Gonzalez, C.; Morales-Hernandez, R.M.; Nunez-Hernandez, J.A.; Hernandez-Quiroz, F.; Romero-Figueroa, M.D.S.; Hernandez-Guerrero, C.; et al. Gut Microbiota and Endothelial Dysfunction Markers in Obese Mexican Children and Adolescents. Nutrients 2018, 10, 2009. [Google Scholar] [CrossRef] [Green Version]
- Jaworek, J.; Tudek, B.; Kowalczyk, P.; Kot, M.; Szklarczyk, J.; Leja-Szpak, A.; Pierzchalski, P.; Bonior, J.; Dembinski, A.; Ceranowicz, P.; et al. Effect of Endotoxemia in Suckling Rats on Pancreatic Integrity and Exocrine Function in Adults: A Review Report. Gastroenterol. Res. Pract. 2018, 2018, 6915059. [Google Scholar] [CrossRef] [Green Version]
- Dembinski, A.; Warzecha, Z.; Ceranowicz, P.; Pawlik, M.; Dembinski, M.; Kabat, K.; Konturek, S.J.; Kownacki, P.; Hladki, W.; Pawlik, W.W. Influence of central and peripheral administration of pancreatic polypeptide on gastric mucosa growth. J. Physiol. Pharmacol. 2004, 55, 223–237. [Google Scholar]
- Warzecha, Z.; Dembinski, A.; Ceranowicz, P.; Stachura, J.; Tomaszewska, R.; Konturek, S.J. Effect of sensory nerves and CGRP on the development of caerulein-induced pancreatitis and pancreatic recovery. J. Physiol. Pharmacol. 2001, 52, 679–704. [Google Scholar] [PubMed]
- O’Brien, R.; O’Malley, D. The Glucagon-like peptide-1 receptor agonist, exendin-4, ameliorated gastrointestinal dysfunction in the Wistar Kyoto rat model of Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2020, 32, e13738. [Google Scholar] [CrossRef] [PubMed]
- Uyar, G.O.; Yildiran, H. A nutritional approach to microbiota in Parkinson’s disease. Biosci. Microbiota Food Health 2019, 38, 115–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagpal, R.; Yadav, H. Bacterial Translocation from the Gut to the Distant Organs: An Overview. Ann. Nutr. Metab. 2017, 71 (Suppl. 1), 11–16. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.L.; Lin, T.L.; Chang, C.J.; Wu, T.R.; Lai, W.F.; Lu, C.C.; Lai, H.C. Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 2019, 26, 3. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.F.; de Oliveira, H.L.; Yamada, E.S.; Neves, B.C.; Pereira, A., Jr. The Gut and Parkinson’s Disease-A Bidirectional Pathway. Front. Neurol. 2019, 10, 574. [Google Scholar] [CrossRef] [Green Version]
- Fang, X. Microbial treatment: The potential application for Parkinson’s disease. Neurol. Sci. 2019, 40, 51–58. [Google Scholar] [CrossRef]
- Cassani, E.; Privitera, G.; Pezzoli, G.; Pusani, C.; Madio, C.; Iorio, L.; Barichella, M. Use of probiotics for the treatment of constipation in Parkinson’s disease patients. Minerva Gastroenterol. Dietol. 2011, 57, 117–121. [Google Scholar]
- Tamtaji, O.R.; Taghizadeh, M.; Daneshvar Kakhaki, R.; Kouchaki, E.; Bahmani, F.; Borzabadi, S.; Oryan, S.; Mafi, A.; Asemi, Z. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 1031–1035. [Google Scholar] [CrossRef]
- Gazerani, P. Probiotics for Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 4121. [Google Scholar] [CrossRef] [Green Version]
- Ekstrand, M.I.; Terzioglu, M.; Galter, D.; Zhu, S.; Hofstetter, C.; Lindqvist, E.; Thams, S.; Bergstrand, A.; Hansson, F.S.; Trifunovic, A.; et al. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 1325–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Redus, L.; Chen, C.; Martinez, P.A.; Strong, R.; Li, S.; O’Connor, J.C. Cognitive dysfunction precedes the onset of motor symptoms in the MitoPark mouse model of Parkinson’s disease. PLoS ONE 2013, 8, e71341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parashar, A.; Udayabanu, M. Gut microbiota: Implications in Parkinson’s disease. Parkinsonism Relat. Disord. 2017, 38, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Yun, M.; Oh, Y.J.; Choi, H.J. Mind-altering with the gut: Modulation of the gut-brain axis with probiotics. J. Microbiol. 2018, 56, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Keller, F.; Emde, C.; Schwarz, A. Exponential function for calculating saturable enzyme kinetics. Clin. Chem. 1988, 34, 2486–2489. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.Q.; Goodrich, G.S.; Dhamne, S.C.; Amandusson, A.; Hsieh, T.H.; Mou, D.; Wang, Y.; Rotenberg, A. A rapid lateral fluid percussion injury rodent model of traumatic brain injury and post-traumatic epilepsy. Neuroreport 2014, 25, 532–536. [Google Scholar] [CrossRef]
- Hsieh, T.H.; Kang, J.W.; Lai, J.H.; Huang, Y.Z.; Rotenberg, A.; Chen, K.Y.; Wang, J.Y.; Chan, S.Y.; Chen, S.C.; Chiang, Y.H.; et al. Relationship of mechanical impact magnitude to neurologic dysfunction severity in a rat traumatic brain injury model. PLoS ONE 2017, 12, e0178186. [Google Scholar] [CrossRef]
- Matter, A.M.; Folweiler, K.A.; Curatolo, L.M.; Kline, A.E. Temporal effects of environmental enrichment-mediated functional improvement after experimental traumatic brain injury in rats. Neurorehabil. Neural Repair 2011, 25, 558–564. [Google Scholar] [CrossRef]
- Onyszchuk, G.; He, Y.Y.; Berman, N.E.; Brooks, W.M. Detrimental effects of aging on outcome from traumatic brain injury: A behavioral, magnetic resonance imaging, and histological study in mice. J. Neurotrauma 2008, 25, 153–171. [Google Scholar] [CrossRef]
- Lai, J.H.; Chen, K.Y.; Wu, J.C.; Olson, L.; Brene, S.; Huang, C.Z.; Chen, Y.H.; Kang, S.J.; Ma, K.H.; Hoffer, B.J.; et al. Voluntary exercise delays progressive deterioration of markers of metabolism and behavior in a mouse model of Parkinson’s disease. Brain Res. 2019, 1720, 146301. [Google Scholar] [CrossRef]
- Liu, N.K.; Zhang, Y.P.; Zou, J.; Verhovshek, T.; Chen, C.; Lu, Q.B.; Walker, C.L.; Shields, C.B.; Xu, X.M. A semicircular controlled cortical impact produces long-term motor and cognitive dysfunction that correlates well with damage to both the sensorimotor cortex and hippocampus. Brain Res. 2014, 1576, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.H.; Chen, J.J.; Chen, L.H.; Chiang, P.T.; Lee, H.Y. Time-course gait analysis of hemiparkinsonian rats following 6-hydroxydopamine lesion. Behav. Brain Res. 2011, 222, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Hsieh, T.H.; Liang, J.I.; Yeh, M.L.; Chen, J.J. Quantitative video-based gait pattern analysis for hemiparkinsonian rats. Med. Biol. Eng. Comput. 2012, 50, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.I.; Lin, P.C.; Chen, M.Y.; Hsieh, T.H.; Chen, J.J.; Yeh, M.L. The effect of tenocyte/hyaluronic acid therapy on the early recovery of healing Achilles tendon in rats. J. Mater. Sci. Mater. Med. 2014, 25, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.L.; Chen, H.Y.; Huang, Y.Z.; Chen, Y.H.; Kuo, C.W.; Chen, K.Y.; Hsieh, T.H. Long-Term Voluntary Physical Exercise Exerts Neuroprotective Effects and Motor Disturbance Alleviation in a Rat Model of Parkinson’s Disease. Behav. Neurol. 2019, 2019, 4829572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaisas, S.; Langley, M.R.; Palanisamy, B.N.; Dutta, S.; Narayanaswamy, K.; Plummer, P.J.; Sarkar, S.; Ay, M.; Jin, H.; Anantharam, V.; et al. MitoPark transgenic mouse model recapitulates the gastrointestinal dysfunction and gut-microbiome changes of Parkinson’s disease. Neurotoxicology 2019, 75, 186–199. [Google Scholar] [CrossRef]
- Ekstrand, M.I.; Galter, D. The MitoPark Mouse—An animal model of Parkinson’s disease with impaired respiratory chain function in dopamine neurons. Parkinsonism Relat. Disord. 2009, 15 (Suppl. 3), S185–S188. [Google Scholar] [CrossRef]
- Barichella, M.; Pacchetti, C.; Bolliri, C.; Cassani, E.; Iorio, L.; Pusani, C.; Pinelli, G.; Privitera, G.; Cesari, I.; Faierman, S.A.; et al. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: An RCT. Neurology 2016, 87, 1274–1280. [Google Scholar] [CrossRef]
- Georgescu, D.; Ancusa, O.E.; Georgescu, L.A.; Ionita, I.; Reisz, D. Nonmotor gastrointestinal disorders in older patients with Parkinson’s disease: Is there hope? Clin. Interv. Aging 2016, 11, 1601–1608. [Google Scholar] [CrossRef] [Green Version]
- Hill-Burns, E.M.; Debelius, J.W.; Morton, J.T.; Wissemann, W.T.; Lewis, M.R.; Wallen, Z.D.; Peddada, S.D.; Factor, S.A.; Molho, E.; Zabetian, C.P.; et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov. Disord. 2017, 32, 739–749. [Google Scholar] [CrossRef]
- Scheperjans, F.; Aho, V.; Pereira, P.A.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 2015, 30, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.B.; Shannon, K.M.; Kordower, J.H.; Voigt, R.M.; Shaikh, M.; Jaglin, J.A.; Estes, J.D.; Dodiya, H.B.; Keshavarzian, A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE 2011, 6, e28032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrucci, D.; Cerroni, R.; Unida, V.; Farcomeni, A.; Pierantozzi, M.; Mercuri, N.B.; Biocca, S.; Stefani, A.; Desideri, A. Dysbiosis of gut microbiota in a selected population of Parkinson’s patients. Parkinsonism Relat. Disord. 2019, 65, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Minato, T.; Maeda, T.; Fujisawa, Y.; Tsuji, H.; Nomoto, K.; Ohno, K.; Hirayama, M. Progression of Parkinson’s disease is associated with gut dysbiosis: Two-year follow-up study. PLoS ONE 2017, 12, e0187307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magistrelli, L.; Amoruso, A.; Mogna, L.; Graziano, T.; Cantello, R.; Pane, M.; Comi, C. Probiotics May Have Beneficial Effects in Parkinson’s Disease: In vitro Evidence. Front. Immunol. 2019, 10, 969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dembinski, A.; Warzecha, Z.; Ceranowicz, P.; Konturek, S.J. The role of capsaicin-sensitive sensory neurons and nitric oxide in regulation of gastric mucosal growth. J. Physiol. Pharmacol. 1995, 46, 351–362. [Google Scholar]
- Dembinski, A.; Warzecha, Z.; Konturek, P.J.; Ceranowicz, P.; Konturek, S.J. Influence of capsaicin-sensitive afferent neurons and nitric oxide (NO) on cerulein-induced pancreatitis in rats. Int. J. Pancreatol. 1996, 19, 179–189. [Google Scholar] [CrossRef]
- Warzecha, Z.; Dembinski, A.; Jaworek, J.; Ceranowicz, P.; Szlachcic, A.; Walocha, J.; Konturek, S.J. Role of sensory nerves in pancreatic secretion and caerulein-induced pancreatitis. J. Physiol. Pharmacol. 1997, 48, 43–58. [Google Scholar]
- Dembinski, A.; Warzecha, Z.; Ceranowicz, P.; Jaworek, J.; Sendur, R.; Knafel, A.; Dembinski, M.; Bilski, J.; Pawlik, W.W.; Tomaszewska, R.; et al. Stimulation of sensory nerves and CGRP attenuate pancreatic damage in ischemia/reperfusion induced pancreatitis. Med. Sci. Monit. 2003, 9, BR418–BR425. [Google Scholar]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Kim, D.Y.; Camilleri, M. Serotonin: A mediator of the brain-gut connection. Am. J. Gastroenterol. 2000, 95, 2698–2709. [Google Scholar] [CrossRef] [PubMed]
- Spohn, S.N.; Mawe, G.M. Non-conventional features of peripheral serotonin signalling—The gut and beyond. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Tatewaki, M.; Yamada, T.; Fujimiya, M.; Mantyh, C.; Voss, M.; Eubanks, S.; Harris, M.; Pappas, T.N.; Takahashi, T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1269–R1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Politis, M.; Niccolini, F. Serotonin in Parkinson’s disease. Behav. Brain Res. 2015, 277, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Arancibia, C.; Urrutia-Pinones, J.; Illanes-Gonzalez, J.; Martinez-Pinto, J.; Sotomayor-Zarate, R.; Julio-Pieper, M.; Bravo, J.A. Do your gut microbes affect your brain dopamine? Psychopharmacology 2019, 236, 1611–1622. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Huang, L.; Li, P.; Zhang, D. Effects of regulating gut microbiota on the serotonin metabolism in the chronic unpredictable mild stress rat model. Neurogastroenterol. Motil. 2019, 31, e13677. [Google Scholar] [CrossRef] [Green Version]
- Srivastav, S.; Neupane, S.; Bhurtel, S.; Katila, N.; Maharjan, S.; Choi, H.; Hong, J.T.; Choi, D.Y. Probiotics mixture increases butyrate, and subsequently rescues the nigral dopaminergic neurons from MPTP and rotenone-induced neurotoxicity. J. Nutr. Biochem. 2019, 69, 73–86. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.F.; Zhu, Y.L.; Zhou, Z.L.; Jia, X.B.; Xu, Y.D.; Yang, Q.; Cui, C.; Shen, Y.Q. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-alpha signaling pathway. Brain Behav. Immun. 2018, 70, 48–60. [Google Scholar] [CrossRef]
- Erickson, J.T.; Brosenitsch, T.A.; Katz, D.M. Brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor are required simultaneously for survival of dopaminergic primary sensory neurons in vivo. J. Neurosci. 2001, 21, 581–589. [Google Scholar] [CrossRef] [Green Version]
- Banks, W.A.; Erickson, M.A. The blood-brain barrier and immune function and dysfunction. Neurobiol. Dis. 2010, 37, 26–32. [Google Scholar] [CrossRef]
- Musa, N.H.; Mani, V.; Lim, S.M.; Vidyadaran, S.; Abdul Majeed, A.B.; Ramasamy, K. Lactobacilli-fermented cow’s milk attenuated lipopolysaccharide-induced neuroinflammation and memory impairment in vitro and in vivo. J. Dairy Res. 2017, 84, 488–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodes, L.; Khan, A.; Paul, A.; Coussa-Charley, M.; Marinescu, D.; Tomaro-Duchesneau, C.; Shao, W.; Kahouli, I.; Prakash, S. Effect of probiotics Lactobacillus and Bifidobacterium on gut-derived lipopolysaccharides and inflammatory cytokines: An in vitro study using a human colonic microbiota model. J. Microbiol. Biotechnol. 2013, 23, 518–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, T.-H.; Kuo, C.-W.; Hsieh, K.-H.; Shieh, M.-J.; Peng, C.-W.; Chen, Y.-C.; Chang, Y.-L.; Huang, Y.-Z.; Chen, C.-C.; Chang, P.-K.; et al. Probiotics Alleviate the Progressive Deterioration of Motor Functions in a Mouse Model of Parkinson’s Disease. Brain Sci. 2020, 10, 206. https://doi.org/10.3390/brainsci10040206
Hsieh T-H, Kuo C-W, Hsieh K-H, Shieh M-J, Peng C-W, Chen Y-C, Chang Y-L, Huang Y-Z, Chen C-C, Chang P-K, et al. Probiotics Alleviate the Progressive Deterioration of Motor Functions in a Mouse Model of Parkinson’s Disease. Brain Sciences. 2020; 10(4):206. https://doi.org/10.3390/brainsci10040206
Chicago/Turabian StyleHsieh, Tsung-Hsun, Chi-Wei Kuo, Kai-Hsuan Hsieh, Meng-Jyh Shieh, Chih-Wei Peng, Yen-Chien Chen, Ying-Ling Chang, Ying-Zu Huang, Chih-Chung Chen, Pi-Kai Chang, and et al. 2020. "Probiotics Alleviate the Progressive Deterioration of Motor Functions in a Mouse Model of Parkinson’s Disease" Brain Sciences 10, no. 4: 206. https://doi.org/10.3390/brainsci10040206