Abstract
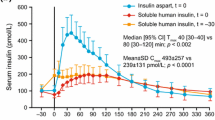
Insulin glargine is a recombinant human insulin analog produced by DNA technology using a nonpathogenic strain of Escherichia coli. Two modifications of human insulin result in a stable molecule which is soluble in slightly acidic conditions (pH 4.0) and precipitates in the neutral pH of subcutaneous tissue. Because of these properties, absorption of insulin glargine is delayed and the analog provides a fairly constant, basal insulin supply without peaks in plasma insulin levels for approximately 24 hours, similar to that achieved by a continuous subcutaneous insulin infusion.
Insulin glargine is indicated as a once daily subcutaneous injection to provide basal glycemic control in adults and children aged >6 years with type 1 diabetes mellitus and in adults with type 2 diabetes mellitus. Fasting plasma glucose and fasting blood glucose levels generally improved to a greater extent in patients with type 1 diabetes mellitus receiving insulin glargine than patients who administered Neutral Protamine Hagedorn (NPH) insulin. In patients with type 1 or 2 disease, glycosylated hemoglobin levels were slightly reduced and to a similar extent with insulin glargine and NPH insulin. Most clinical trials in patients with type 1 or 2 diabetes mellitus demonstrated a lower incidence of hypoglycemia, especially nocturnal hypoglycemia, with insulin glargine compared with NPH insulin.
One of the most common adverse events with insulin glargine treatment was injection site pain which, in some studies, occurred more frequently than in patients receiving NPH insulin. In all cases the symptoms were mild and treatment discontinuation was not required. Otherwise, the drug is well tolerated and does not appear to be immunogenic.
In conclusion, insulin glargine once a day provides basal control of glycemia for approximately 24 hours without inducing peaks in plasma insulin levels in patients with type 1 or 2 diabetes mellitus. In long-term, well designed trials insulin glargine once daily improved glycemic control at least as effectively as NPH insulin given once or twice daily. The drug was well tolerated and in most studies the incidence of nocturnal hypoglycemia was significantly less in patients treated with insulin glargine compared with patients receiving NPH insulin. Therefore, insulin glargine is likely to be a useful addition to the armamentarium of insulin therapy by establishing basal glycemic control with once daily administration and a reduced risk of nocturnal hypoglycemia.
Similar content being viewed by others
References
Bolli GB, Di Marchi RD, Park GD, et al. Insulin s and their potential in the management of diabetes mellitus. Diabetologia 1999 Oct; 42: 1151–67
Home P. Insulin glargine: the first clinically useful extended-acting insulin in half a century? Expert Opin Invest Drug 1999 Mar; 8: 307–14
Mohn A, Dunger DB. Insulin therapy in children and adolescents with type 1 diabetes. Curr Paediatr 1999; 9: 158–63
Talaulicar M, Willms B, Rosskamp R. HOE 901, a new insulin, for substitution of basal insulin requirement in type I diabetes [in German]. Diabetes Stoffwechsel 1996; 5: 3–6
Heinemann L, Linkeschova R, Rave K, et al. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care 2000 May; 23: 644–9
Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes 2000 Dec; 49: 2142–8
Meyer BH, Scholtz HE, Pretorius SG, et al. A comparison of the pharmacodynamics (glucose lowering effect) of intravenous HOE901 and regular insulin using the euglycaemic clamp technique [abstract]. Clin Pharmacol Ther 2000 Feb; 67: 123
Mohideen P, Mudaliar S, Deutsch R, et al. Characterization of glucose turnover of insulin glargine in comparison with regular human insulin in healthy male subjects [abstract no. 879]. 35th Annual Meeting of the European Association for the Study of Diabetes (EASD); 1999 28 Sep–2 Oct; Brussels. Diabetologia 1999 Sep/Oct; 42Suppl. 1: A234
Dagogo-Jack S, Askari H, Morrill B, et al. Physiological responses during hypoglycaemia induced by regular human insulin or a novel human, insulin glargine. Diabetes Obes Metab 2000; 2: 373–83
Dagogo-Jack S, Askari H, Lehner LL. Effect of glargine on glucose disposal and lipolysis in healthy and diabetic subjects [abstract 1757]. Diabetes 2000 May; 49Suppl. 1: A412
Seipke G, Berchthold H, Geisen K, et al. HOE901 — a new insulin with prolonged action [abstract]. Eur J Endocrinol 1995 Apr; 132Suppl. 1: 25
Bähr M, Kolter T, Seipke G, et al. Growth promoting and metabolic activity of the human insulin [GlyA21,ArgB31,ArgB32] insulin (HOE 901) in muscle cells. Eur J Pharmacol 1997 Feb 12; 320: 259–65
Kurtzhals P, Schaffer L, Sorensen A, et al. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 2000 Jun; 49: 999–1005
Sandow J, Seipke G. In vitro pharmacology studies with insulin glargine and human insulin: IGF-1 receptor binding and thymidine incorporation [abstract no. 1787-PO]. Diabetes 2001 Jun; 50Suppl. 2: A429
Stammberger I, Troschau G, Donaubauer H. Insulin glargine is not carcinogenic in rats and mice [abstract no. 1788-PO]. Diabetes 2001 Jun; 50Suppl. 2: A429
Luzio SD, Owens D, Evans M, et al. Comparison of the sc absorption of HOE 901 and NPH human insulin type 2 diabetic subjects [abstract]. Diabetes 1999 May; 48Suppl. 1: 111
Owens DR, Coates PA, Luzio SD, et al. Pharmacokinetics of 125I-labeled insulin glargine (HOE 901) in healthy men. Diabetes Care 2000 Jun; 23: 813–9
Heise T, Bott S, Rave K, et al. No evidence for accumulation of HOE901 after multiple injections in type I diabetic patients. Diabetologia 2000; 43Suppl. 1: A196
Seipke G, Sandow J, Geisen K, et al. Preclinical profile of the new long-acting insulin glargine (HOE 901) [abstract no. Pl-283]. 82nd Annual Meeting of the Endocrine Society; 2000 Jun 21–24; Toronto (ON)
Sandow J, Seipke G, Kuerzel GU, et al. Pharmacokinetis and metabolism of insulin glargine [abstract no. P1-358]. Endocrine Society 83rd Annual Meeting; 2001 Jun 20–23; Denver (CO)
Hershon K, Blevins T, Donley D, et al. Lower fasting blood glucose (FBG) and less symptomatic hypoglycemia with QD insulin glargine (Lantus) compared to BID NPH in subjects with type 1 diabetes [abstract 466-P]. Diabetes 2001 Jun; 50Suppl. 2: A116
Pieber TR, Eugène-Jolchine I, Derobert E, et al. Efficacy and safety of HOE 901 versus NPH insulin in patients with type I diabetes. Diabetes Care 2000; 23(2): 157–62
Raskin P, Klaff L, Bergenstal R, et al. A 16-week comparison of the novel insulin analog insulin glargine (HOE 901) and NPH human insulin used with insulin lispro in patients with type 1 diabetes. Diabetes Care 2000 Nov; 23: 1666–71
Ratner RE, Hirsch IB, Neifing JL, et al. Less hypoglycemia with insulin glargine in intensive insulin therapy for type 1 diabetes. Diabetes Care 2000 May; 23: 639–43
Rosenstock J, Park G, Zimmerman J, et al. Basal insulin glargine (HOE 901) versus NPH insulin in patients with type 1 diabetes on multiple daily insulin regimens. Diabetes Care 2000 Aug; 23: 1137–42
Standl E. Results of an international, multicentred, randomised 28 week study for the comparison of glargine insulin (HOE 901) and NPH insulin in the intensified treatment (ICT) of Type 1 Diabetics [abstract no. pFr1O7]. Exp Clin Endocrinol Diabetes 2000; 108Suppl. 1: 159
Schoenle E. Insulin glargine (HOE 901) lowers fasting blood glucose in children with type I diabetes mellitus without increasing the risk of hypoglycaemia [abstract no. 883]. Diabetologia 1999; 42Suppl. 1: A235
Fonseca V, Bell D, Mecca T. Less symptomatic hypoglycemia with bedtime insulin glargine (Lantus) compared to bedtime NPH insulin in patients with type 2 diabetes [abstract no. 449-P]. Diabetes 2001 Jun; 50Suppl. 2: A112
Massi Benedetti M. Results of a 12 month study: insulin glargine (HOE 901) in combination with oral agents (pre-EASD Symposium, Brussels) [abstract]. European Association for the Study of Diabetes; 1999 Sep; Brussels.
Matthews DR, Pfeiffer C. Comparative clinical trial of a new long-acting insulin (HOE901) vs. protamine insulin demonstrates less nocturnal hypoglycaemia. Multicentre HOE901 Research Group [abstract]. Diabetes 1998 May; 47Suppl. 1: A101
Raskin P, Park G, Zimmerman J. The effect of HOE 901 on glycemic control in type 2 diabetes [abstract]. Diabetes 1998 May; 47Suppl. 1: A103
Rosenstock J, Schwartz SL, Clark C, et al. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care 2001 Apr; 24(4): 631–6
Yki-Jarvinen H, Dressier A, Ziemen M, et al. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. Diabetes Care 2000 Aug; 23: 1130–6
Witthaus E, Stewart J, Bradley C. Treatment satisfaction and psychological well-being with insulin glargine compared with NPH in patients with type 1 diabetes. Diabetic Med 2001 Aug; 18(8): 619–25
Witthaus E, Stewart J, Bradley C. Improved psychological outcomes after initiation of insulin treatment in patients with type II diabetes. Diabetologia 2000; 43Suppl. 1: A205
Working Group. HOE 901 Retinopathy Expert Statement [Data on file]. 1999 Aug 5
Aventis Pharmaceuticals Inc. Prescribing information: insulin glargine [rDNA origin] injection. Kansas City (KS): Aventis Pharmaceuticals Inc., 2000 Apr
AHFS Drug Information. Insulin glargine. Am J Health System Pharm 2000 Nov 1;57: 1961
Acknowledgements
The full text of this article appeared in Drugs 2001; 61 (11): 1599–1624, and was reviewed by: G. Bolli,, University of Perugia, Perugia, Italy; S. Dagogo-Jack, Department of Medicine (Endocrinology), University of Mississippi Medical Center, Jackson, Mississippi, USA; L. Heinemann, Profil Institute for Metabolic Research, Neuss, Germany; P.D. Home, Department of Medicine, University of Newcastle upon Tyne, Newcastle upon Tyne, England; R. Ratner, Medstar Research Institute, Washington, DC, USA; D.L. Trence, Diabetes Care Center, University of Washington Medical Center, Seattle, Washington, USA; H. Yki-Jarvinen, Department of Medicine, University of Helsinki, Helsinki, Finland.
Author information
Authors and Affiliations
Corresponding author
Additional information
The full text of this article appeared in Drugs 2001; 61 (11): 1599–1624
Rights and permissions
About this article
Cite this article
McKeage, K., Goa, K.L. Spotlight on Insulin Glargine in Type 1 and 2 Diabetes Mellitus. Mol Diag Ther 1, 55–58 (2002). https://doi.org/10.2165/00024677-200201010-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00024677-200201010-00006