Summary
The aim of this study was to determine the incremental effectiveness, the incremental health-related quality of life (differences in quality-adjusted progression-free survival between treatments), the incremental cost and the incremental cost-effectiveness and cost-utility ratios, for docetaxel, paclitaxel and vinorelbine, when these drugs were used as second-line treatment in patients with metastatic breast cancer.
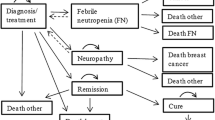
In the absence of comparative direct evidence of the relative efficacy of docetaxel, paclitaxel and vinorelbine in this setting, a model was designed to determine the effects of the 3 interventions on health outcome and cost. A Markov process model, based on 53 disease states, was thus constructed to evaluate the socioeconomics of the 3 treatment regimens.
The model allows assessments from the start of second-line chemotherapy until death. Costs were evaluated from the combined view of the healthcare system and the patient. Direct nonmedical and indirect costs were excluded. Consumption per episode of care was estimated by retrospective analysis of 153 medical reports from 5 different hospitals. Hospital costs were allocated values from the national accounting costs by diagnosis-related group (DRG). The content of the health states was based on the multiattribute health states classification system (MASH). Preference values were assigned by application of a standard reference lottery using 20 oncological nurses as proxies for the patients. The health-related quality-of-life score was used as a quality adjustment weighting factor to calculate quality-adjusted progression-free survival associated with the 3 different regimens.
Docetaxel reduces the time spent in progression, decreases the number of complications due to progressive disease and thereby provides better quality of life. It provides a benefit of 57 disease- and discomfort-free days compared with vinorelbine and 22 days compared with paclitaxel.
Docetaxel may be thought of as self-financing as a result of savings in hospital admissions, providing net savings of 6800 French francs (FF; 1993 values) compared with expenditure associated with vinorelbine treatment and FF700 compared with the equivalent figures for paclitaxel.
Similar content being viewed by others
References
Benhamou E, Laplanche A, Wartelle M, et al. Incidence des cancers en France 1978–1982. Statistiques de Santé. Paris: Editions INSERM, 1990
Hill C, Benhamou E, Doyon F, et al. Evolution de la mortalité par cancer en France entre 1950 et 1985. Statistiques de Santé. Paris: Editions INSERM, 1989
Fédération Nationale des Centres de Lutte contre le Cancer. Enquete permanente cancer 1975/1986–monographic des cancers du sein. Paris: Edition Doin, 1991
Genot JY. Cancer du sein — surveillance post-thérapeutique. Paris: Centre d’Information Régionale sur le Cancer (CIRCAN), 1994
Smith TJ, Hillner BE, Desch E. Efficacy and cost-effectiveness of cancer treatment: rational allocation of resources based on decision analysis. J Nat Cancer Inst 1993; 85 (18): 1460–74
Hillner BE, Smith TJ. Efficacy and cost-effectiveness of adjuvant chemotherapy in women with node-negative breast cancer: a decision-analysis model. N Engl J Med 1991; 324 (3): 160–8
Hillner BE, Smith TJ. A model of chemotherapy in node-negative breast cancer. J Nat Cancer Inst Monogr 1992; 11: 143–9
Hillner BE, Smith TJ, Desch CE. Efficacy and cost-effectiveness of autologous bone marrow transplantation in metastatic breast cancer: estimates using decision analysis while awaiting clinical trial results. JAMA 1992; 267 (15): 2055–61
Eddy DM. High-dose chemotherapy with autologous bone marrow transplantation for the treatment of metastatic breast cancer. J Clin Oncol 1992; 10 (4): 657–70
Reboul-Marty J, Henry B, Aussage P, et al. Metastatic breast cancer management in France: a representative survey [abstract]. Pharmacoepidemiol Drug Saf 1996: 5 Suppl. 1: S70
Beck RJ, Pauker SG. The Markov process in medical prognosis. Med Decis Making 1983; 3: 419–58
Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making 1993; 13: 322–38
Degardin M, Bonneterre J, Hecquet B, et al. Vinorelbine as a salvage treatment for advanced breast cancer. Ann Oncol 1994; 5: 423–6
Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer 1981; 47: 207–14
Hollenberg J. SMLTREE: the all-purpose decision tree builder. Boston: Pratt Medical Group, 1993: 76
Nabholtz JM, Gelmon K, Bontenbal M, et al. Randomized trial of two doses of paclitaxel in metastatic breast cancer: an interim analysis [abstract no. 42]. Proc Am Soc Clin Oncol 1993; 12: 60
Food and Drug Administration Center for Drug Evaluation and Research Oncologic Drugs Advisory Committee. Study 048: multicentric randomized study of two doses of taxol in metastatic breast cancer. 048F01-F017.CH3, 1993
Docetaxel centralised procedure no. 73. Registration dossier. Part IV. Clinical documentation and updated expert report 1995. Committee for Proprietary Medicinal Products - European Agency for the evaluation of Medicinal Products, 1995
Ten Bokkel Huinink WW, Prove AM, Picard M, et al. A phase II trial with docetaxel in second line treatment with chemotherapy for advanced breast cancer: a study of the EORTC Early Clinical Trials Group. Ann Oncol 1994; 5 (6): 527–32
Valero V, Holmes FA, Walters RS, et al. Phase II trial of docetaxel: a new, highly effective antineoplastic agent in the management of patients with anthracycline-resistant metastatic breast cancer. J Clin Oncol 1995; 13: 2886–94
Ravdin PM, Burris HA, Cook G, et al. Phase II trial of docetaxel in advanced anthracycline-resistant or anthracenedione-resistant breast cancer. J Clin Oncol 1995; 13: 2879–85
Nabholtz JM, Gelmon K, Bontenbal M, et al. Multicenter, randomized comparative study of two doses of paclitaxel in patients with breast cancer. J Clin Oncol 1996; 14: 1858–67
Kleinbaum DG, Kupper LL, Mogenstern H. Epidemiologic research: principles and quantitative methods. London: Lifetime Learning Publications, 1982: 529
Miller DK, Homan SM. Determining transition probabilities: confusion and suggestions. Med Decis Making 1994; 14: 52–8
Beck JR, Pauker SG, Gottlieb JE, et al. A convenient approximation of life expectancy (the DEALE). II: Use in medical decision making. Am J Med 1982; 73: 889–97
Nomenclature générate des actes professionnels. Paris: UCANSS, 1993: 107
Nomenclature des actes de biologie médicale. Paris: UCANSS, 1993: 106
Programme de médicalisation des systèmes d’information (PMSI). Les groupes homogènes de malades (GHM). Paris: Ministère des Affaires Sociales et de l’Emploi. Bulletin Officiel no. 86-30 bis, 1986
Fascicule spécial PMSI-Manuel des GHM version 1. Paris: Ministère des Affaires Sociales et de la Solidarité Nationale. Bulletin Officiel no. 92-9 bis, 1992
Classification Internationale des Maladies. 9th revision. Vols 1 and 2. Geneva: WHO, 1977
L’échelle nationale des coûts relatifs par Groupe Homogène de Malades. Paris: Ministerè des Affaires Sociales, de la Santé et de la Ville. Bulletins Officiels no. 95-5 bis, 1995
Guide méthodologique de comptabilité analytique hospitalière. Calcul des couts des stuctures hospitalieres. Paris: Ministère des Affaires Sociales et de la Solidarité Nationale. Bulletins Officiels no. 88-14 bis, 1988
Guide méthodologique de comptabilité analytique hospitalière. Calcul des couts de revient complet par Groupe Homogène de malades. Paris: Ministère des Affaires Sociales et de la Solidarité Nationale. Bulletins Officiels no. 85-26 bis, 1985
Launois R. Quality of life: overview and perspectives. Drug Info J 1994; 28: 123–40
Feeny D, Furlong W, Boyle M, et al. Multi-attribute health status classification systems: health utilities index. Pharmacoeconomics 1995; 7 (6): 490–502
Feeny D, Furlong W, Barr RD, et al. A comprehensive multi-attribute system for classifying the health status of survivors of childhood cancer. J Clin Oncol 1992; 10 (6): 923–8
Furlong W, Feeny D, Torrance GW, et al. Guide to design and development of health-state utility instrumentation. CHEPA Working Paper Series no. 90-9. Ontario: McMaster University, 1990: 140
Torrance GW. Social preferences for health states: an empirical evaluation of the three measurement techniques. Socioecon Planning Sci 1976; 10: 129–36
Dieras V, Marty M, Morvan F, et al. Essai de phase II randomisant taxol versus mitomycin dans le cancer du sein metastase en 2eme ligne [abstract]. Analyse Intermediate Bulletin du Cancer 1994; 81: 450
Seidman AD, Tiersten A, Hudis C, et al. Phase II trial of paclitaxel by 3-hour infusion as initial and salvage chemotherapy for metastatic breast cancer. J Clin Oncol 1995; 13: 2575–81
Launois R, Orvain J, Ounis I. Apport d’une mesure des utilites: infections respiratoires recidivates. Rev Epidem et Santé Publ 1992; 40: 46–55
Clarridge BR, Massagli MP. The use of female spouse proxies in common symptom reporting. Med Care 1989; 4: 639–48
Magaziner J, Simonsick EM, Kashner TM, et al. Patient proxy response comparability on measures of patient health status and functional status. J Clin Epidemiol 1988; 41: 1065–74
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Launois, R., Reboul-Marty, J., Henry, B. et al. A Cost-Utility Analysis of Second-Line Chemotherapy in Metastatic Breast Cancer. Pharmacoeconomics 10, 504–521 (1996). https://doi.org/10.2165/00019053-199610050-00008
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-199610050-00008