Abstract
Background: Academic medical centres face the need to care for patients with complex medical conditions, educate physicians-in-training and conduct research, all with increasingly constrained budgets. The adoption of new therapeutic technology presents challenges and opportunities in each of these areas. Severe sepsis remains a major cause of morbidity and mortality, especially in tertiary-care facilities. Recombinant human activated protein C reduces mortality in patients with severe sepsis, but trial data indicate that the benefit of the drug is confined to the more seriously ill patients, while the risk of bleeding complications can be considerable. The cost of the drug is approximately $US6000–8000 per treated patient. Integration of this product into routine care has produced unique challenges concerning clinical decision making, safety and cost.
Objectives: To describe one hospital’s multidisciplinary approach to the adoption of this new medication.
Methods: Before activated protein C was approved for use, Brigham and Women’s Hospital (BWH) convened a working group to formulate clinical guidelines proactively. This new agent did not fit into an obvious therapeutic category but cut across multiple clinical disciplines requiring the involvement of several hospital departments in developing policy. As new data on efficacy emerged during the US FDA review of the drug, the working group had to devise a method for using the available information to assist clinical decision making while placing appropriate restrictions on the use of activated protein C. The goal was to make accurate information available to guide ordering physicians’ decision making interactively, 24 hours a day.
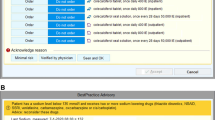
Results: The committee developed a utilisation policy for activated protein C that provided guidance on patient selection, contraindications and risk stratification. Interactive computer-based order entry screens were developed to guide physicians through a complex set of clinical criteria to ensure appropriate evidence-based use. A careful review of contraindications is required as a second step. To risk stratify patients in accordance with the trial subset analyses and the FDA labelling guidelines, ordering physicians are guided in calculating an APACHE II (Acute Physiology and Chronic Health Evaluation) score for the patient. Physicians from several specialties are available for advice and consultation on patients with difficult or controversial conditions. Approximately two-thirds of completed orders passed the clinical algorithm; an additional 35% of patients did not meet the medication criteria but received the drug after the attending physician requested an override of the guidelines.
Conclusion: The BWH approach to activated protein C used an innovative multidisciplinary approach and computer-assisted order entry to guide clinical use of a new agent with substantial clinical efficacy, risks and costs. This approach provides a model for strategies to deal with other new and complex medical technologies.





Similar content being viewed by others
Notes
1 The use of trade names is for product identification purposes only and does not imply endorsement.
References
Blumenthal D, Campbell EG, Weissman JS. The social missions of academic health centers. N Engl J Med 1997; 337: 1550–3
Linde-Zwirble WT, Angus DC, Carcillo J, et al. Age-specific incidence and outcome of sepsis in the US. Crit Care Med 1999; 27: 33A
American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. Definitions for sepsis and multiple organ failure, and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992; 20: 864–74
Rangel-Frausto MS, Pittet D, Costigan M, et al. The natural history of the systemic inflammatory response syndrome (SIRS): a prospective study. JAMA 1995; 273: 117–23
Sands KE, Bates DW, Lanken P, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA 1997; 278: 234–40
Astiz ME, Rackow EC. Septic shock. Lancet 1998; 351: 1501–5
Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 2001; 344: 699–709
Friedman G, Silva E, Vincent JL. Has the mortality of septic shock changed with time? Crit Care Med 1998; 26: 2078–86
Anti-Infective Drugs Advisory Committee, US FDA. Drotrecogin alfa (activates) [recombinant human activated protein C (rhAPC)]: briefing information. September 12, 2001 [online]. Available from URL: http://www.fda.gov/ohrms/dockets/ac/01/briefing/3787b1.htm [Accessed 2004 Sep 23]
Ely E, Laterre P, Angus D. Drotrecogin alfa (activated) administration across clinically important subgroups of patients with severe sepsis. Crit Care Med 2003; 31: 12–9
Knaus W, Draper E, Wagner D, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818–29
Bernard G. Drotrecogin alfa (activated) (recombinant human activated protein C) for the treatment of severe sepsis. Crit Care Med 2003; 31: S85–93
Wong D, Yang P. Managing high-cost biotechnology products. Am J Health Syst Pharm 2003; 60: 1213–6
Shah N, Hoffman J, Vermeulen L, et al. Projecting future drug expenditures: 2003. Am J Health Syst Pharm 2003; 60: 137–49
Siegel JP. Assessing the use of activated protein C in the treatment of severe sepsis. N Engl J Med 2002; 347: 1030–4
Approval letter: drotrecogin alfa (activated). Rockville (MD): US FDA, 2001
Drotrecogin alfa (activated), Xigris: product label. Indianapolis (IN): Eli Lilly and Co., 2001
European Agency for the Evaluation of Medicinal Products. Committee for Proprietary Medicinal Products: 28–30 May 2002 plenary meeting monthly report [online]. Available from URL: http://www.emea.eu.int/pdfs/human/press/pr/222202-en.pdf [Accessed 2004 Nov 4]
Levi M. Benefit of recombinant human activated protein C beyond 28-day mortality: there is more to life than death. Crit Care Med 2003; 31: 984–5
Mathiak G, Neville L, Grass G. Targeting the coagulation cascade in sepsis: did we find the ‘magic bullet’? Crit Care Med 2003; 31: 310–1
Vincent J, Angus D, Artigas A, et al. Effects of drotrecogin alfa (activated) on organ dysfunction in the PROWESS trial. Crit Care Med 2003; 31: 834–40
Eichacker P, Natanson C. Recombinant human activated protein C in sepsis: inconsistent trial results, an unclear mechanism of action, and safety concerns result in labeling restrictions and the need for phase IV trials. Crit Care Med 2003; 31: S94–6
Warren HS, Suffredini AF, Eichacker PQ, et al. Risks and benefits of activated protein C treatment for severe sepsis. N Engl J Med 2002; 347: 1027–30
Teich JM, Glaser JP, Beckley RF, et al. The Brigham integrated computing system (BICS): advanced clinical systems in an academic hospital environment. Int J Med Inf 1999; 54: 197–208
Applebaum S, Carter C, Chuen T, et al. Patient selection guidelines and DUE for drotrecogin alfa (activated). Hosp Formul 2002; 37: 3–15
Vanscoy G, Devlin J, Ponzillo J, et al. Implementing guidelines for drotrecogin alfa (activated): three perspectives. P&T 2002; 27: 2–13
Manns BJ, Lee H, Doig CJ, et al. An economic evaluation of activated protein C treatment for severe sepsis. N Engl J Med 2002; 347: 993–1000
Angus D, Linde-Zwirble W, Clermont G, et al. Cost-effectiveness of drotrecogin alfa (activated) in the treatment of severe sepsis. Crit Care Med 2003; 31: 1–11
Banks S, Gerstenberger E, Eichacker Q, et al. Long-term cost effectiveness of drotrecogin alfa (activated): an unanswered question. Crit Care Med 2003; 31: 308–9
Chalfin D, Teres D, Rapoport J. A price for cost-effectiveness: implications for recombinant human activated protein C (rhAPC). Crit Care Med 2003; 31: 306–8
Thompson C. Medicare to partly cover extra cost of sepsis therapy, drug-eluting stents. Am J Health Syst Pharm 2002; 59: 1816–7
Centers for Medicare and Medicaid Services, Department of Health and Human Services (DoHaHS). Medicare program: changes to the hospital inpatient prospective payment system and fiscal year 2003 rates. 42 C.F.R. pt 405, 50013-50016. Final rule: 67 Fed. Reg. 148 (2002)
Acknowledgements
This study was financially supported by Brigham and Women’s Hospital. The authors gratefully acknowledge assistance from Danielle Cabral, Edna Marston and Gene Lysen.
Michael A. Fischer MD, MS has no financial or personal conflicts of interest to report. Craig M. Lilly, MD has received a research grant from Eli Lilly but was not receiving any funding from Eli Lilly at the time that the guidelines were created. Lindsey R. Baden, MD has no financial or personal conflicts of interest to report. William W. Churchill, MS, RPh has served on a customer advisory board for Eli Lilly. Jerry Avorn, MD has no financial or personal conflicts of interest to report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fischer, M.A., Lilly, C.M., Churchill, W.W. et al. An Algorithmic Computerised Order Entry Approach to Assist in the Prescribing of New Therapeutic Agents. Drug-Safety 27, 1253–1261 (2004). https://doi.org/10.2165/00002018-200427150-00008
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200427150-00008