Abstract
Osteogenesis imperfecta is a heritable condition characterized by abnormally brittle bones, with an approximate prevalence of 1/20 000 births. Fractures are the main cause of suffering and disability, but owing to the abundance and wide distribution of the defective type I collagen in the body, a variety of symptoms occur. Several types of osteogenesis imperfecta (I-VII) have been described that vary in severity. For many years, therapy consisted of rehabilitation and orthopedic surgery. Presently, pharmacologic therapies aimed at strengthening bone are available, which decrease the pain and fracture rate associated with this condition, and allow more appropriate rehabilitation programs that will hopefully result in a less marked failure to thrive in affected children. In particular, the bisphosphonates, especially pamidronate, have been used for several years. They have been successful in increasing bone mineral density (BMD) and improving bone resistance, leading to a decrease in the fracture rate. Various regimens have been proposed, but it is the therapeutic regimen first used by Glorieux and co-workers in Montreal that has been the most frequently applied.
However, as yet there is no definite consensus regarding the indications for therapy, the osteogenesis imperfecta types that are of the greatest concern, the appropriate age at the outset of therapy, and the treatment duration, without yet speaking about the best bisphosphonate regimen for use. The authors have proposed some personal recommendations for the clinical use of bisphosphonates, based on their own experience with the management of patients with this condition; these include the indications for therapy, based on the clinical status, and the treatment duration. These recommendations will certainly not be unanimously endorsed, but they should help to stimulate discussion. Ameliorating BMD is an important step, but will not prevent all fractures because bisphosphonate therapy does not correct the underlying genetic defect. More recently, stem cell replacement therapy in the child or fetus has been proposed as a therapeutic option.
All in all, it is possible that, in order to dramatically decrease the fracture rate, combined therapies aimed at both circumventing the consequences of the gene defect using stem cells and reinforcing bone strength with bisphosphonates will have to be considered. Much work is still necessary before recommending these techniques in clinical practice.






Similar content being viewed by others
References
Sillence DO. Osteogenesis imperfecta: an expanding panorama of variants. Clin Orthop 1981; 159: 11–25
Byers PH. Brittle bones: fragile molecules: disorders of collagen gene structure and expression. Trends Genet 1990; 6: 293–300
Ward LM, Lalic L, Roughley PJ, et al. Thirty-three novel COL1 A1 and COL1 A2 mutations in patients with osteogenesis imperfecta types I—IV. Hum Mutat 2001; 17: 434–9
Database of human type I and type III collagen mutations [online]. Available from URL: http://www.le.ac.uk/genetics/collagen [Accessed 2006 Jul 18]
Dalgleish R. The human type I collagen mutation database. Nucleic Acids Res 1997; 25: 181–7
Dalgleish R. The human collagen mutation database. Nucleic Acids Res 1998; 26: 253–5
Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet 1979; 16: 101–16
Glorieux FH, Rauch F, Plotkin H, et al. Type V osteogenesis imperfecta: a new form of brittle bone disease. J Bone Miner Res 2000; 15: 1650–8
Glorieux FH, Ward LM, Rauch F, et al. Osteogenesis imperfecta type VI: a form of brittle bone disease with a mineralization defect. J Bone Miner Res 2002; 17: 30–8
Ward LM, Rauch F, Travers R, et al. Osteogenesis imperfecta type VII: an autosomal recessive form of brittle bone disease. Bone 2002; 31: 12–8
Labuda M, Morissette J, Ward LM, et al. Osteogenesis imperfecta type VII maps to the short arm of chromosome 3. Bone 2002; 31: 19–25
Byers PH, Steiner RD. Osteogenesis imperfecta. Annu Rev Med 1992; 43: 269–82
Roughley PJ, Rauch F, Glorieux FH. Osteogenesis imperfecta: clinical and molecular diversity. Eur Cell Mater 2003; 5: 41–7
Pierog SH, Fontana VJ, Ferrara A. Osteogenesis imperfecta: therapeutic challenge. N Y State J Med 1969; 69: 310–3
Zanzi I, Wallach S, Ellis KJ, et al. Long-term treatment of osteogenesis imperfecta tarda in adults with salmon calcitonin and calcium. Curr Ther Res Clin Exp 1976; 19: 189–97
Hoekman K, Papapoulos SE, Peters ACB, et al. Characteristics and bisphosphonate treatment of a patient with juvenile osteoporosis. J Clin Endocrinol Metab 1985; 61: 952–6
Baron R, Gertner JM, Lang R, et al. Increased bone turnover with decreased bone formation by osteoblasts in children with osteogenesis imperfecta tarda. Pediatr Res 1983; 17: 204–7
Devogelaer JP, Malghem J, Maldague B, et al. Radiological manifestations of bisphosphonate treatment with APD in a child suffering from osteogenesis imperfecta. Skeletal Radiol 1987; 16: 360–3
Jones SJ, Glorieux FH, Travers R, et al. The microscopic structure of bone in normal children and patients with osteogenesis imperfecta: a survey using backscattered electron imaging. Calcif Tissue Int 1999; 64: 8–17
Rauch F, Travers R, Parfitt AM, et al. Static and dynamic bone histomorphometry in children with osteogenesis imperfecta. Bone 2000; 26: 581–9
Boyde A, Travers R, Glorieux FH, et al. The mineralization density of iliac crest bone from children with osteogenesis imperfecta. Calcif Tissue Int 1999; 64: 185–90
Boivin G, Chavassieux P, Forin V, et al. Effects of pamidronate on bone modeling, bone remodeling and degree of mineralization of bone from children with osteogenesis imperfecta [abstract]. J Bone Miner Res 2005; 20 Suppl. 1: S24
Rauch F, Land C, Cornibert S, et al. High and low density in the same bone: a study on children and adolescents with mild osteogenesis imperfecta. Bone 2005; 37: 634–41
Bonjour J-PH, Theintz G, Buchs B, et al. Critical years and stages of puberty for spinal and femoral bone mass accumulation during adolescence. J Clin Endocrinol Metab 1991; 73: 555–63
Theintz G, Buchs B, Rizzoli R, et al. Longitudinal monitoring of bone mass accumulation in healthy adolescents: evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. J Clin Endocrinol Metab 1992; 75: 1060–5
Eastell R, Adams JE, Ahmed SF, et al. A practical guide to bone densitometry in children. Bath: National Osteoporosis Society, 2004 Nov
Szulc P, Seeman E, Delmas PD. Biochemical measurements of bone turnover in children and adolescents. Osteoporos Int 2000; 11: 281–4
Russell RGG, Croucher PI, Rogers MJ. Bisphosphonates: pharmacology, mechanisms of action and clinical uses. Osteoporos Int 1999; 9 Suppl. 2: S66–80
Luckman SP, Hughes DE, Coxon FP, et al. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res 1998; 13: 581–9
Nancollas GH, Tang R, Phipps RJ, et al. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 2006; 38(5): 617–27
Devogelaer J-P. New uses of bisphosphonates: osteogenesis imperfecta. Curr Opin Pharmacol 2002; 2: 748–53
Engelbert RH, Pruijs HE, Beemer FA, et al. Osteogenesis imperfecta in childhood: treatment strategies. Arch Phys Med Rehabil 1998; 79: 1590–4
Glorieux FH, Bishop NJ, Plotkin H, et al. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med 1998; 339: 947–52
Plotkin H, Rauch F, Bishop NJ, et al. Pamidronate treatment of severe osteogenesis imperfecta in children under 3 years of age. J Clin Endocrinol Metab 2000; 85: 1846–50
Rauch F, Plotkin H, Travers R, et al. Osteogenesis imperfecta types I, III, and IV: effect of pamidronate therapy on bone and mineral metabolism. J Endocrinol Metab 2003; 88: 986–92
Rauch F, Plotkin H, Zeitlin L, et al. Bone mass, size, and density in children and adolescents with osteogenesis imperfecta: effect of intravenous pamidronate therapy. J Bone Miner Res 2003; 18: 610–4
Falk MJ, Heeger S, Lynch KA, et al. Intravenous bisphosphonate therapy in children with osteogenesis imperfecta. Pediatrics 2003; 111: 573–8
DiMeglio LA, Ford L, McClintock C, et al. Intravenous pamidronate treatment of children under 36 months of age with osteogenesis imperfecta. Bone 2004; 35: 1038–45
Zeitlin L, Rauch F, Travers R, et al. The effect of cyclical intravenous pamidronate in children and adolescents with osteogenesis imperfecta type V. Bone 2006; 38: 13–20
Montpetit K, Plotkin H, Rauch F, et al. Rapid increase in grip force after start of pamidronate therapy in children and adolescents with severe osteogenesis imperfecta. Pediatrics 2003; 111: 601–3
Arikoski P, Silverwood B, Tillmann V, et al. Intravenous pamidronate treatment in children with moderate to severe osteogenesis imperfecta: assessment of indices of dual-energy x-ray absorptiometry and bone metabolic markers during the first year of therapy. Bone 2004; 34: 539–4
Munns CFJ, Rauch F, Travers R, et al. Effects of intravenous pamidronate treatment in infants with osteogenesis imperfecta: clinical and histomorphometric outcome. J Bone Miner Res 2005; 20: 1235–43
Rauch F, Travers R, Plotkin H, et al. The effects of intravenous pamidronate on the bone tissue of children and adolescents with osteogenesis imperfecta. J Clin Invest 2002; 110: 1293–9
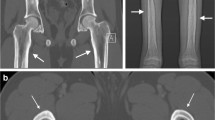
van Persijn van Meerten EL, Kroon HM, Papapoulos SE. Epi- and metaphyseal changes in children caused by administration of bisphosphonates. Radiology 1992; 184: 249–54
Devogelaer JP, Nagant de Deuxchaisnes C. Use of pamidronate in chronic and acute bone loss conditions. Medicina 1997; 57 Suppl. 1: 101–8
Devogelaer JP, Nagant de Deuxchaisnes C, Malghem J, et al. Cyclical intermittent therapy with APD in a child with osteogenesis imperfecta: a 3-year follow-up of 7 cycles: evidence of fading away of the oldest radio-opaque metaphyseal bands. In: Christiansen C, Overgaard K, editors. Osteoporosis 1990. Aalborg: Handelstrykkeriet Aalborg ApS, 1990: 1515–7
Rauch F, Travers R, Munns C, et al. Sclerotic metaphyseal lines in a child treated with pamidronate: histomorphometric analysis. J Bone Miner Res 2004; 19: 1191–3
Fuchs RK, Peacock M, McClintock C, et al. Children with osteogenesis imperfecta treated with bisphosphonates have site-specific bone responses to therapy [abstract]. J Bone Miner Res 2005; 20 Suppl. 1: S302
DiMeglio LA, Ford L, McClintock C, et al. A comparison of oral and intravenous bisphosphonate therapy for children with osteogenesis imperfecta. J Pediatr Endocrinol Metab 2005; 18: 43–53
Glorieux FH, Plotkin H, Chiodo III J, et al. A randomized, open-label, comparison of zoledronic acid and pamidronate treatment in children with severe osteogenesis imperfecta [abstract]. Bone 2005; 36 Suppl. 1: S81
Whyte MP, Wenkert D, Clements KL, et al. Bisphosphonate-induced osteopetrosis. N Engl J Med 2003; 349: 457–63
Astrom E, Soderhall S. Beneficial effect of bisphosphonate during five years of treatment of severe osteogenesis imperfecta. Acta Paediatr 1998; 87: 64–8
Astrom E, Soderhall S. Beneficial effects of long term intravenous bisphosphonate treatment of osteogenesis imperfecta. Arch Dis Child 2002; 86: 356–64
Anton J, Ricart S, Ros J, et al. Cyclic pamidronate in children with osteogenesis imperfecta: review of a protocol of a single-day infusion twice a year [abstract]. Bone 2005; 36 Suppl. 1: S82
Bembi B, Parma A, Bottega M, et al. Intravenous pamidronate treatment in osteogenesis imperfecta. J Pediatr 1997; 131: 622–5
Gatti D, Viapiana O, Lippolis I, et al. Intravenous bisphosphonate therapy increases radial width in adults with osteogenesis imperfecta. J Bone Miner Res 2005; 20: 1323–6
Adami S, Gatti D, Colapietro F, et al. Intravenous neridronate in adults with osteogenesis imperfecta. J Bone Miner Res 2003; 18: 126–30
Gatti D, Antoniazzi F, Prizzi R, et al. Intravenous neridronate in children with osteogenesis imperfecta: a randomized controlled study. J Bone Miner Res 2005; 20: 758–63
Landsmeer-Beker EA, Massa GG, Maaswinkel-Mooy PD, et al. Treatment of osteogenesis imperfecta with the bisphosphonate olpadronate (dimethylaminohydroxypropylidene bisphosphonate). Eur J Pediatr 1997; 156: 792–4
Sakkers R, Kok D, Engelbert R, et al. Skeletal effects and functional outcome with olpadronate in children with osteogenesis imperfecta: a 2-year randomised placebo-controlled study. Lancet 2004; 363: 1427–31
Vyskocil V, Pikner R, Kutilek S. Effect of alendronate therapy in children with osteogenesis imperfecta. Joint Bone Spine 2005; 72: 416–23
Ward LM, Glorieux FH, Rauch F, et al. A randomized placebo-controlled trial of oral alendronate children and adolescents with osteogenesis imperfecta [abstract]. Bone 2005; 36 Suppl. 1: S31
Chevrel G, Schott A-M, Fontanges E, et al. Effects of oral alendronate on BMD in adult patients with osteogenesis imperfecta: a 3-year randomized placebocontrolled trial. J Bone Miner Res 2006; 21: 300–6
Gensure RC, Ponnapakkam T. Treatment of osteogenesis imperfecta type I with weekly alendronate [abstract]. J Bone Miner Res 2005; 20 Suppl. 1: S175
Senthilnathan S, Walker E, Cousins R, et al. Pamidronate for infants with osteogenesis imperfecta: comparison of different doses [abstract]. Bone 2005; 36 Suppl. 1: S82
Letocha AD, Cintas HL, Troendle JF, et al. Controlled trial of pamidronate in children with type III and IV osteogenesis imperfecta confirms vertebral gains but not short-term functional improvement. J Bone Miner Res 2005; 20: 977–86
Land C, Rauch F, Montpetit K, et al. Effect of intravenous pamidronate therapy on functional ability and degree of ambulation in children with osteogenesis imperfecta [abstract]. Bone 2005; 36 Suppl. 1: S32
Ward LM, Denker AE, Porras A, et al. Single-dose pharmacokinetics and tolerability of alendronate 35- and 70-milligram tablets in children and adolescents with osteogenesis imperfecta type I. J Clin Endocrinol Metab 2005; 90: 4051–6
Devogelaer JP, Depresseux G. Estimation from early changes of serum procollagen type 1 aminoterminal propeptide of the lumbar BMD gain after 2 years in children suffering from severe osteogenesis imperfecta [abstract]. Bone 2005; 36 Suppl. 1: S41
Schweitzer DH, Oostendorp-van de Ruit M, van der Pluijm G, et al. Interleukin-6 and the acute phase response during treatment of patients with Paget’s disease with the nitrogen-containing bisphosphonate dimethylaminohydroxypropylidene bisphosphonate. J Bone Miner Res 1995; 10: 956–62
Thiebaud D, Sauty A, Burckhardt P, et al. An in vitro and in vivo study of cytokines in the acute-phase response associated with bisphosphonates. Calcif Tissue Int 1997; 61: 386–92
Thompson K, Rogers MJ. Statins prevent bisphosphonate-induced gamma, delta-T-cell proliferation and activation in vitro. J Bone Miner Res 2004; 19: 278–88
Lufkin EG, Argueta R, Whitaker MD, et al. Pamidronate: an unrecognized problem in gastrointestinal tolerability. Osteoporos Int 1994; 4: 320–2
Macarol V, Fraunfelder FT. Pamidronate disodium and possible ocular adverse drug reactions. Am J Ophthalmol 1994; 118: 220–4
Ruguiero SL, Mehrotra B, Rosenberg TJ, et al. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 2004; 62: 527–34
Munns CF, Rauch F, Mier RJ, et al. Respiratory distress with pamidronate treatment in infants with severe osteogenesis imperfecta. Bone 2004; 35: 231–4
Munns CFJ, Rauch F, Zeitlin L, et al. Delayed osteotomy but not fracture healing in pediatric osteogenesis imperfecta patients receiving pamidronate. J Bone Miner Res 2004; 19: 1779–86
Rauch F, Land C, Munns C, et al. The effect of pamidronate discontinuation in children and adolescents with moderate to severe osteogenesis imperfecta [abstract]. J Bone Miner Res 2005; 20 Suppl. 1: S23
Rauch F, Travers R, Glorieux FH. Pamidronate in children with osteogenesis imperfecta: histomorphometric effects of long-term therapy. J Clin Endocrinol Metab 2006; 91: 511–6
McClung MR, Lewiecki EM, Cohen SB, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 2006; 354: 821–31
Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001; 344: 1434–41
Black DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 2003; 349: 1207–15
Finkelstein JS, Hayes A, Hunzelman JL, et al. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med 2003; 349: 1216–26
Black DM, Bilezikian JP, Ensrud KE, et al. One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med 2005; 353: 555–65
Antoniazzi F, Bertoldo F, Mottes M, et al. Growth hormone treatment in osteogen- esis imperfecta with quantitative defect of type I collagen synthesis. J Pediatr 1996; 129: 432–9
Marini JC, Hopkins E, Glorieux FH, et al. Positive linear growth and bone responses to growth hormone treatment in children with types III and IV osteogenesis imperfecta: high predictive value of the carboxy-terminal propeptide of type I procollagen. J Bone Miner Res 2003; 18: 237–43
Horwitz EM, Prockop DJ, Gordon PL, et al. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood 2001; 97: 1227–31
Niyibizi C, Wallach CJ, Mi Z, et al. Approaches for skeletal gene therapy. Crit Rev Eukaryot Gene Expr 2002; 12: 163–73
Chamberlain JR, Schwarze U, Wang P-R, et al. Gene targeting in stem cells from individuals with osteogenesis imperfecta. Science 2004; 303: 1198–201
Pochampally RR, Horwitz EM, Digirolamo CM, et al. Correction of a mineralization defect by overexpression of a wild-type cDNA for COL1A1 in marrow stromal cells (MSCs) from a patient with osteogenesis imperfecta: a strategy for rescuing mutations that produce dominant-negative protein defects. Gene Ther 2005; 12: 1119–25
Le Blanc K, Gotherstrom C, Ringden O, et al. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation 2005; 79: 1607–14
Acknowledgments
We are grateful to Marie-Christine Hallot for her helpful assistance in the typing of the manuscript. No sources of funding were used to assist in the preparation of this review. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Devogelaer, JP., Coppin, C. Osteogenesis Imperfecta. Mol Diag Ther 5, 229–242 (2006). https://doi.org/10.2165/00024677-200605040-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00024677-200605040-00004