Abstract
Background:The combination of intravenous paclitaxel (three-times weekly administration at a dose of 175 mg/m2) and oral capecitabine has been shown to be highly active in the treatment of advanced or metastatic breast cancer. Currently, there is much interest in the use of relatively low dose weekly paclitaxel infusions in this clinical setting.
Aim:To assess the activity and safety of capecitabine plus weekly paclitaxel at a dose of 60 mg/m2in heavily pretreated metastatic breast cancer patients.
Patients and methods:Patients were required to have pretreated metastatic breast cancer with measurable/ evaluable disease and a performance status of 0–2 (Eastern Cooperative Oncology Group score). Capecitabine was administered orally at a dosage of 1000 mg/m2twice daily on days 1–14, followed by a 7-day rest period. Paclitaxel was administered as a 1-hour intravenous infusion on a weekly basis at a dose of 60 mg/m2. Twenty-one days of therapy represented one cycle.
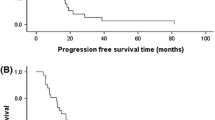
Results:The hypothesis of activity of the capecitabine-paclitaxel combination at the level of clinical interest (40% response rate) was accepted when the 15th response was observed and 33 patients were enrolled. The median progression-free survival and median overall survival estimates were 9.2 and 19.6 months, respectively. Therapy was generally well tolerated and manageable on an outpatient basis. The following grade 3/4 adverse events were observed: hand-foot syndrome (21.2%), neutropenia (12.1%), anemia, nausea/vomiting, stomatitis/ mucositis, and nail disorders (all occurred in 9.0%), and vascular/coagulation disorders (<1%).
Conclusions:Oral capecitabine plus weekly paclitaxel at a dose of 60 mg/m2has a favorable activity and tolerability profile and is suitable for the treatment of heavily pretreated advanced breast cancer patients.



Similar content being viewed by others
References
Perez EA. Current management of metastatic breast cancer. Semin Oncol 1999; 26(12 Suppl.): 1–10
Hortobagyi GN. Progress in systemic chemotherapy of primary breast cancer: an overview. J Natl Cancer Inst Monogr 2001; 30: 72–9
Michaud LB, Valero V, Hortobagyi G. Risks and benefit of taxanes in breast and ovarian cancer. Drag Saf 2000; 23: 401–28
Miwa M, Ura M, Nishida M, et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer 1998; 34: 1274–81
Sawada N, Ishikawa T, Fukase Y, et al. Induction of thymidine phosphorylase activity and enhancement of capecitabine efficacy by taxol/taxotere in human cancer xenografts. Clin Cancer Res 1998; 4: 1013–9
Ishitsuka H. Capecitabine: preclinical pharmacology studies. Invest New Drugs 2000; 18: 343–54
Blum JL, Dieras V, Lo Russo PM, et al. Multicenter, phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer 2001; 92: 1759–68
Liekens S, DeClercq E, Neyts J. Angiogenesis regulators and clinical applications. Biochem Pharmacol 2001; 61: 253–70
Villalona-Calero MA, Blum JL, Jones SE, et al. A phase I and pharmacologic study of capecitabine and paclitaxel in breast cancer patients. Ann Oncol 2001; 12: 605–14
Gradishar WJ, Meza LA, Amin B, et al. Capecitabine plus paclitaxel as front-line combination therapy for metastatic breast cancer: a multicenter phase II study. J Clin Oncol 2004; 22: 2321–7
Batista N, Perez-Manga G, Constenla M, et al. Phase II study of capecitabine in combination with paclitaxel in patients with anthracycline-pretreated advanced/ metastatic breast cancer. Br J Cancer 2004; 90: 1740–6
Maher JF, Villalona-Calero MA. Taxanes and capecitabine in combination: rationale and clinical results. Clin Breast Cancer 2002; 2: 287–93
Perez EA, Vogel CL, Irwin DH, et al. Multicenter phase II trial of weekly paclitaxel in women with metastatic breast cancer. J Clin Oncol 2001; 19: 4216–23
Uhlmann C, Ballabeni P, Rijken N, et al. Capecitabine with weekly paclitaxel for advanced breast cancer: a phase I dose-finding trial. Oncology 2004; 67: 117–22
Seidman AD, Berry D, Cirrincione L, et al. Phase III study of weekly (W) paclitaxel (P) via l-hour (h) infusion versus standard (S) 3h infusion every third week in the treatment of metastatic breast cancer (MBC), with trastuzumab (T) for HER2 positive MBC and randomized to T in HER2 normal MBC [abstract no. 512]. J Clin Oncol ASCO Annual Meeting Proceedings (Post-Meeting Edition) 2004July 15; 22(14S Suppl.): 512
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16
Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989; 10: 1–10
Ishikawa T, Utoh M, Sawada N, et al. Tumor selective delivery of 5-fluorouracil by capecitabine, a new oral fluoropyrimidine carbamate, in human cancer xenografts. Biochem Pharmacol 1998; 55: 1091–7
Judson IR, Beale PJ, Trigo JM, et al. A human capecitabine excretion balance and pharmacokinetic study after administration of a single oral dose of 14C labelled drug. Invest New Drugs 1999; 17: 49–56
Blum JL, Jones SE, Buzdar AU, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol 1999; 17: 485–93
Fumoleau P, Largillier R, Trillet-Lenoir V, et al. Phase II study of capecitabine (Xeloda®) in pts with advanced breast cancer (ABC), previously treated with anthracyclines and taxanes. Breast Cancer Res Treat 2001; 69: 285a
Reichardt P, VonMinckwitz G, Lück HJ, et al. Multicenter phase II study of oral capecitabine (Xeloda®) in patients with metastatic breast cancer relapsing after treatment with a taxane-containing therapy. Ann Oncol 2003Aug; 14(8): 1227–33
Blum J, Jones S, Buzdar A. Capecitabine (Xeloda®) in 162 patients with paclitaxel-pretreated mbc: updated results and analysis of dose modification. Eur J Cancer 2001; 37(6 Suppl.): S190a
Talbot DC, Moiseyenko V, VanBelle S, et al. Randomised, phase II trial comparing oral capecitabine (Xeloda®) with paclitaxel in patients with metastatic/ advanced breast cancer pretreated with anthracyclines. Br J Cancer 2002; 86: 1367–72
O’Shaughnessy J, Miles D, Vukelja S, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol 2002; 20: 2812–23
Acknowledgments
Capecitabine was provided by Roche, Italy. No sources of funding were used to plan and conduct this study. The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bari, M., D’Andrea, M.R., Azzarello, G. et al. Salvage Therapy with Capecitabine Plus Weekly Paclitaxel in Heavily Pretreated Advanced Breast Cancer. Am J Cancer 4, 307–313 (2005). https://doi.org/10.2165/00024669-200504050-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00024669-200504050-00003