Summary
In the absence of comparative clinical and pharmacoeconomic trial data for docetaxel versus paclitaxel as second-line therapy for patients with anthracycline-resistant metastatic breast cancer, a computer-based decision-analysis model was designed to evaluate the comparative utility to patients of these two taxoids. The model used the Markov process to analyse disease states (response, stable disease, progressive disease) and toxicities (acute, cumulative) for each treatment during the period from commencement of up to six 3-weekly cycles of chemotherapy, to death. A cost-utility analysis was carried out using the model, with a probability, a cost and a utility determined for each health state. Response rates were obtained from clinical trial data supplemented by expert clinical opinion. Costs were taken from UK national databases and published sources and the published UK prices of docetaxel and paclitaxel. Utilities for the various health states were established by use of standard gamble and visual analogue methods assessed by 30 oncology nurses in the UK who were acting as proxy patients.
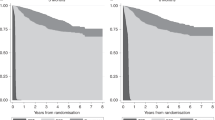
The results of the model showed that response rate is the key parameter determining the utility and cost utility of treatments for metastatic breast cancer. Although the total per-patient cost associated with docetaxel was marginally higher than that for paclitaxel (£8233 vs £8013), the higher response rate associated with docetaxel produced an improvement in utility to the patient at an incremental healthcare cost that is acceptable according to available defined limits. Sensitivity analyses revealed that, although the model was sensitive to changes in response rate and drug costs, the cost-utility ratio for docetaxel versus paclitaxel varied within acceptable limits in response to all likely changes in key parameters. In summary, in the base case used in this model, docetaxel produces a substantially larger utility benefit than paclitaxel, at a small additional cost per QALY gained (equivalent to £7 per additional day of perfect health).
Similar content being viewed by others
References
Drummond M, Futten F, Brenna A, et al. Economic evaluation of pharmaceuticals: a European perspective. PharmacoEconomics 1993; 4(3): 173–86
Williams C, Coyle D, Uyl-de-Groot C, et al. European School of Oncology Advisory Report to the Commission of the European Communities for the ‘Europe Against Cancer Programme’ Cost-effectiveness in Cancer Care. Eur J Cancer 1995; 31A: 1410–24
Torrance GW. Utility approach to measuring health-related quality of life. J Chron Dis 1987; 40: 593
Mouridsen HT. Systemic therapy of advanced breast cancer. Drugs 1992; 44 Suppl. 4: 17–28
Henderson IC. Chemotherapy for metastatic disease. In Harris JR, Hellman S, Henderson IC, et al., editors. Breast diseases. 2nd ed. Philadelphia: JB Lippincott, 1991: 604–5
Jones S, Winer E, Vogel C, et al. Randomised comparison of vinorelbine and melphalan in anthracycline-refractory advanced breast cancer. J Clin Oncol 1995; 13: 2567–74
Vandenberg TA. New developments in chemotherapy for metastatic breast cancer. Anti-Cancer Drugs 1994; 5: 251–9
ten Bokkel Huinink WW, Prove AM, Piccart M, et al. A phase II trial with docetaxel (Taxotere®) in second line treatment with chemotherapy for advanced breast cancer. A study of the EORTC Early Clinical Trials Group. Ann Oncol 1995; 5(6): 527–32
Chevallier B, Fumoleau P, Kerbrat P, et al. Docetaxel is a major cytotoxic drug for the treatment of advanced breast cancer: a phase II trial of the Clinical Screening Cooperative Group of the European Organisation for Research and Treatment of Cancer. J Clin Oncol 1995; 13: 314–22
Hudis CA, Seidman AD, Crown JPA, et al. Phase II pharmacologic study of docetaxel as initial chemotherapy for metastatic breast cancer. J Clin Oncol 1996; 14: 58–65
Trudeau ME, Eisenhauer E, Higgins BP, et al. Docetaxel in patients with metastatic breast cancer: a phase II study of the National Institute of Canada-Clinical Trials Group. J Clin Oncol 1996; 14: 422–8
Ravdin PM, Burris HA III, Cook G, et al. Phase II trial of docetaxel in advanced anthracycline-resistant or anthracenedione-resistant breast cancer. J Clin Oncol 1995; 13: 2879–85
Valero V, Holmes FA, Walters RS, et al. Phase II trial of docetaxel: a new, highly effective antineoplastic agent in the treatment of patients with anthracycline-resistant metastatic breast cancer. J Clin Oncol 1995; 13: 2886–94
Guastalla JP, Bonneterre J, Fumoleau P, et al. A phase II trial of docetaxel in patients (pts) with anthracycline resistant (AR) metastatic breast cancer (MBC) [abstract]. 8th European Conference on Clinical Oncology and Cancer Nursing (ECCO), Paris, 29 Oct–2 Nov, 1995
Hillner BE, Smith TJ. Efficacy and cost effectiveness of adjuvant chemotherapy in women with node-negative breast cancer. N Engl J Med 1991; 324(3): 160–8
Hillner BE, Smith TJ. Should women with node-negative breast cancer receive adjuvant chemotherapy? — Insights from a decision analysis model. Breast Cancer Res Treat 1992; 23: 17–27
Epstein RJ. Does the breast cancer dollar make sense? Eur J Cancer 1992; 28(2): 486–91
Richards MA, Braysher S, Gregory WM, et al. Advanced breast cancer: use of resources and cost implications. Br J Cancer 1993; 67: 856–60
de Koning HJ, van Ineveld BM, de Haes JC, et al. Advanced breast cancer and its prevention by screening. Br J Cancer 1992; 65: 950–5
Hurley SF, Huggins RM, Snyder RD, et al. The cost of breast cancer recurrences. Br J Cancer 1992; 65: 445–9
Desch CE, Hillner BE, Smith TJ. Should the elderly receive chemotherapy for node-negative breast cancer? A cost-effectiveness analysis examining total and active life expectancy outcomes. J Clin Oncol 1993; 11(4): 777–82
Seidman A. Quality of life in metastatic breast cancer. The impact of taxol. Comparisons between FLIG, rand general well-being, hand function. McCorcle and Young, MSAS/GOI. BMS
Cochrane WG. The combination of estimates from different experiments. Biometrics 1954; 10: 101–29
Louis TA, Fineburg H, Mosteller F. Findings for public health from meta-analysis. Annual reviews of public health. Palo Alto, CA: Annual Reviews Inc., 1985: 1–20
Summary of Product Characteristics for Taxotere® (docetaxel). Rhône-Poulenc Rorer, November 1995
Taxotere® Prescribing Information, US Datasheet 1996
Seidman AD, Tiersten A, Hudis C, et al. Phase II trial of paclitaxel by 3-hour infusion as initial and salvage chemotherapy for metastatic breast cancer. J Clin Oncol 1995; 13: 2575–81
Abrams JS, Vena DA, Baltz J, et al. Paclitaxel activity in heavily pretreated breast cancer: a National Cancer Institute Treatment Referral Center Trial. J Clin Oncol 1995; 13: 2056–65
Seidman AD, Reichman BS, Crown JPA, et al. Paclitaxel as second and subsequent therapy for metastatic breast cancer: activity independent of prior anthracycline response. J Clin Oncol 1995; 13: 1152–9
Taxol® Prescribing Information Datasheet, 1996
Rothman ML, Hedrick SC, Bulcroft KA, et al. The validity of proxy-generated scores as measures of patient health status. Med Care 1991; 29: 115
Furlong W, Feeny D, Torrance GW. Guide to design and development of health state utility instrumentation. Hamilton, Ontario: McMaster University, 1990
Froberg DG, Kane RL. Methodology for measuring health-state preferences. III. Population and context effects. J Clin Epidemiol 1989; 42(c): 585–92
NHS Executive 1994. Hospital and Community Health Services Revenue (Pay and Prices) Inflation Index
MIMS. Monthly Index of Medical Specialties. London: Haymarket Publishing Services Ltd: May 1996
Chartered Institute Public Finance Accountants. Health Database. Health Care Financial Management Association. London: 1992, 1994
Drummond M, Davies L. Economic evaluation of lenograstin for prophylaxis of chemotherapy-induced neutropenia in patients with small cell lung cancer. PharmacoEconomics 1994; 6 Suppl. 2: 44–52
Review body for nursing staff, midwives, health visitors and professions allied to medicine. 11th Report on nursing staff, midwives and health visitors, 1994. CM 2462. HMSO, London: 1994
Review body on doctors’ and dentists’ remuneration. 23rd report, 1994. CM 2460. HMSO, London: 1994
West Midlands Regional Health Authority. Extra-contractual referrals tariff. 1992/3
Leese B. The costs of treating febrile neutropenia in six UK hospitals. Eur J Cancer 1993; 29A Suppl. 7: S15–S18
Department of Health and Welsh Office. Drug Tariff. National Health Service, England and Wales. HMSO, London: June 1994
Gerard K, Mooney G. QALY league tables. Handle with care. Health Econ 1993; 2: 59–64
Laupacis A, Feen D, Detsky A, et al. How attractive does a technology have to be to warrant adoption and utilisation. Can Med Assoc J 1992; 146: 473–81
Detsky A. Formulary 1995; 30
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hutton, J., Brown, R.E., Horowitz, M. et al. A New Decision Model for Cost-Utility Comparisons of Chemotherapy in Recurrent Metastatic Breast Cancer. Pharmacoeconomics 9 (Suppl 2), 8–22 (1996). https://doi.org/10.2165/00019053-199600092-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-199600092-00004