Summary
Synopsis
Parnaparin is a low molecular weight (LMW) heparin which, like other members of its class, apparently demonstrates a greater antithrombotic effect relative to its anticoagulant activity when compared with the unfractionated heparin (heparin) from which it is derived. Moreover, subcutaneous parnaparin has a greater bioavailability and longer half-life than heparin, permitting once-daily administration for the prophylaxis of deep venous thrombosis (DVT) or the treatment of established vascular disorders
Prophylaxis with a 7-day regimen of parnaparin 3200 or 6400 IUaXa/day has consistently been associated with a lower incidence of confirmed DVT compared with usual prophylactic regimens of heparin. This intertreatment difference reached statistical significance in a large multicentre study involving a total of 610 surgical patients (3.2% for parnaparin vs 6.3% for heparin). Thus far, however, comparisons of parnaparin with other LMW heparins for this indication are unavailable.
Parnaparin has demonstrated equivalent efficacy to heparin in the treatment of established vascular disorders, including phlebopathies and related syndromes, as well as peripheral arterial occlusive disease. Parnaparin also showed some benefit as an adjunctive therapy in patients with angina pectoris.
The risk of general bleeding appears to be similar with parnaparin or heparin, although parnaparin results in fewer haematomas at the site of injection, partly because of the less frequent administration regimen. Parnaparin has also been associated with a lower incidence of pain and/or burning sensation at the injection site compared with heparin.
As with other LMW heparins, the possibility that parnaparin will be infrequently associated with thrombocytopenia cannot be excluded.
Thus, parnaparin may be preferred over traditional heparin for the prophylaxis of thrombo-embolic events in surgical patients (particularly those at high risk for DVT), as well as the treatment of established vascular disorders with a thrombotic aetiology. Compared with heparin, parnaparin offers the advantages of a more convenient administration regimen coupled with improved local tolerability. However, the therapeutic advantages of parnaparin relative to other LMW heparins have yet to be established in large scale comparative trials.
Pharmacodynamic Properties
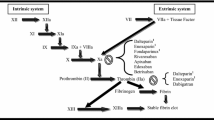
Parnaparin is a low molecular weight (LMW) heparin with a mean molecular weight of 4.5kD. Like other members of its class, parnaparin has been developed because it apparently demonstrates a greater antithrombotic effect relative to its anticoagulant activity when compared with the unfractionated heparin (heparin) from which it is derived
In rodents, parnaparin has demonstrated antithrombotic activity equivalent or superior to that of heparin and a lesser prohaemorrhagic effect.
Tested doses of parnaparin ≤12 800 IUaXa produce dose-dependent inhibition of factor Xa, although there is some interstudy variation in the duration of action. In fact, significant inhibition of factor Xa activity has lasted for up to 24 hours following subcutaneous administration of parnaparin 6400 IUaXa in healthy volunteers. In a comparative study, parnaparin 6400 IUaXa produced inhibition of factor Xa that was both greater and longer lasting than that following heparin 10 000IU. At the same time, parnaparin had a lesser antithrombin effect than heparin, with a smaller increase in the activated partial thromboplastin time (aPTT). The peak increase in aPTT with parnaparin appears to be of minimal clinical significance, even at the highest dose tested (12 800 IUaXa).
Parnaparin is significantly less potent than heparin in potentiating human platelet aggregation induced with agonists, and retains a significantly higher anticoagulant activity in the presence of activated platelets.
The in vitro aPTT activity of parnaparin could be fully neutralised with protamine salts, although the anti-factor Xa (anti-Xa) activity was only partially neutralised, even at the highest protamine dose tested (100 αg/L).
Pharmacokinetic Properties
As with other LMW heparins, the pharmacokinetic properties of parnaparin have been determined indirectly by measurement of anti-Xa activity in the plasma
Compared with subcutaneous administration, the maximal anti-Xa activity is approximately 5-fold higher following intravenous administration of parnaparin. Nonetheless, the subcutaneous bioavailability of parnaparin is estimated to be more than 90%; maximal anti-Xa activity is reached 2 to 4 hours post-injection. Parnaparin bioavailability is independent of the site of subcutaneous administration and similar to that for the intramuscular route.
During repeated subcutaneous administration, a constant level of anti-Xa activity is reached within 2 to 4 days. Once-daily administration results in minimal accumulation of parnaparin beyond the first day, even at the highest dose tested (12 800 IUaXa). However, the likelihood of a cumulative effect may be increased with a twice-daily regimen of parnaparin.
Parnaparin, in common with other LMW heparins, is eliminated primarily by a nonsaturable renal mechanism. Following intravenous administration, plasma clearance was around 0.03 L/h/kg, independent of the tested dose. In contrast, the plasma half-life increased dose-dependently, reaching 134 minutes at the highest dose evaluated (3200 IUaXa).
The pharmacokinetic profile of parnaparin in patients with hepatic or renal failure has yet to be evaluated.
Clinical Efficacy
In clinical trials, subcutaneous administration of parnaparin has proven effective for the prevention of postsurgical deep venous thrombosis (DVT) and pulmonary embolism. Typically, patients at relatively low risk for DVT have received parnaparin 3200 IUaXa/day, while patients at high risk for DVT have received parnaparin 6400 IUaXa/day administered once or twice daily. Parnaparin 6400 IUaXa/day is also effective in maintaining the patency of prosthetic conduits used during arterial repair
In comparative studies, the incidence of confirmed DVT with parnaparin tended to be lower than with standard prophylactic regimens of heparin. In the largest multicentre study, DVT developed in significantly fewer patients receiving parnaparin 3200 or 6400 IUaXa/day compared with patients receiving heparin 10 000 or 15000 IUaXa/day [3.2% (n = 308) vs 6.3% (n = 302)]. In this study, the incidence of pulmonary embolism was also lower with parnaparin treatment (0.3 vs 1 %), although this difference did not reach statistical significance.
Parnaparin, typically administered at a maintenance dosage of 4250 or 6400 IUaXa/day, significantly improved objective measures of venous haemodynamics, as well as subjective assessments of symptoms in patients with various phlebopathies and related syndromes. In comparative trials, parnaparin demonstrated efficacy equivalent to that of heparin ≤25 000 IU/day. Parnaparin 4250 IUaXa/day was also as effective as heparin 15 000 IU/day in providing relief from symptoms associated with chronic venous insufficiency.
Compared with placebo, parnaparin 6400 IUaXa/day has significantly improved objective measures of arterial function as well as subjective assessments of clinical symptoms in patients with peripheral arterial occlusive disease. In a single study, 6 to 8 months’ treatment with parnaparin demonstrated efficacy equivalent to that of heparin 10 000 IU/day.
When investigated as an adjunct to conventional therapy, 3 months’ treatment with parnaparin 6400 IUaXa/day significantly increased the ischaemic threshold in patients with stable angina pectoris.
Tolerability
Parnaparin, in common with other LMW heparins, has the potential to cause an increase in bleeding as an extension of its antithrombotic effect. As expected, meta-analysis reveals a direct relationship between the frequency of haemorrhagic-like episodes and parnaparin dose. However, there is little evidence to suggest a marked increase in the risk of perioperative blood loss and need for transfusion with therapeutic dosages of parnaparin 3200 or 6400 IUaXa/day
The risk of perioperative haemorrhage with parnaparin appears to be similar to that for standard prophylactic regimens of heparin, although comparative trials may have been of insufficient size to reveal a statistically significant difference (if any) between treatments. In the largest trial, clinically detectable haemorrhages occurred in 1% of general surgery patients receiving parnaparin 3200 or 6400 IUaXa/day for 7 days, compared with 4% of patients receiving heparin 10 000 or 15 000 IU/day.
The local tolerability of parnaparin is generally superior to that of heparin, as evidenced by the appearance of significantly fewer haematomas at the injection site, even after correcting for the lower administration frequency with parnaparin. In addition, parnaparin has been associated with a lower incidence of pain and/or burning sensation at the injection site, although the overall incidence is low with both treatments.
Neither heparin-sensitive clotting assays (e.g. aPTT) nor common laboratory parameters are affected to a clinically significant extent by parnaparin.
Though rare, thrombocytopenia has been reported in association with several commercially available LMW heparins. Thrombocytopenia has not been reported to date in clinical trials of parnaparin, although clinical experience with the drug is, as yet, limited.
Dosage and Administration
In patients at relatively low risk of developing DVT, a single subcutaneous injection of parnaparin 3200 IUaXa is usually given 2 hours prior to surgery. Injections are then repeated every 24 hours for at least 7 days. In clinical trials, patients at higher risk of developing DVT, such as those undergoing orthopaedic surgery, have received parnaparin 6400 IUaXa/day administered in a once or twice daily regimen. The manufacturer, however, recommends that parnaparin 4250 IUaXa be administered 12 hours before and after surgery, followed by a daily injection for a minimum of 10 days in high risk patients
Patients with phlebopathies and related syndromes have typically received a maintenance dosage of parnaparin 4250 or 6400 IUaXa/day for up to 3 months, if required. In comparison, patients with peripheral vascular disease of arterial occlusive origin have received parnaparin 6400 IUaXa/day for 6 to 8 months.
Anti-Xa activity should be carefully monitored in patients with renal dysfunction, as parnaparin clearance may be reduced in these circumstances.
Similar content being viewed by others
References
Abildgaard U, Lindahl AK, Sandset PM. Heparin requires both antithrombin and extrinsic pathway inhibitor for its anticoagulant effect in human blood. Haemostasis 21: 254–257, 1991
Agrati AM, Ambrosi G, Spinelli G, Palmieri G, Palazzini E. Influence of the administration site on the bioavailability of a new low molecular weight heparin administered by subcutaneous route. Aggiornamenti di Medicina e Chirurgia 9: 375–379, 1991
Alfa Wassermann SpA. Pamaparin prescribing information, Bologna, Italy, 1993
Anderson DR, O’Brien BJ, Levine MN, Roberts R, Wells PS, et al. Efficacy and cost of low-molecular weight heparin compared with standard heparin for the prevention of deep vein thrombosis after total hip arthroplasty. Annals of Internal Medicine 119: 1105–1112, 1993
Andriuoli G, Mastacchi R, Barbanti M, Sarret M. Comparison of the antithrombotic and haemorrhagic effects of heparin and a new low molecular weight heparin in rats. Haemostasis 15: 324–330, 1985
Anon. Prevention of venous thrombosis and pulmonary embolism. Journal of the American Medical Association 256: 744–749, 1986
Barbanti M, Mastacchi R. The effect of heparin and OP/LMWH on the release of plasminogen activator in perfused rat hind legs. Abstract. Thrombosis and Haemostasis 58: 554, 1987
Barradell LB, Buckley MM. Nadroparin Calcium: a review of its pharmacology and clinical applications in the prevention and treatment of thromboembolic disorders. Drugs 44: 858–888, 1992
Benedetti-Valentini F, Irace L, Gattuso R, Ciocca F, Aracu A, et al. Arterial repair of the lower limbs: prevention of prosthetic grafts occlusion by LMW-heparin. International Angiology 7 (Suppl. 3): 29–32, 1988
Bonomo GM, Scattarella M, Treglia AL. Preventing postsurgery thromboembolism by a new molecular weight heparin. Medical Praxis 9: 375–381, 1988
Buckley MM, Sorkin EM. Enoxaparin: a review of its pharmacology and clinical applications in the prevention and treatment of thromboembolic disorders. Drugs 44: 465–497, 1992
Calabrò A, Piarulli F, Milan D, Rossi A, Coscetti G, et al. Clinical assessment of low molecular weight heparin effects in peripheral vascular disease. Angiology 44: 188–195, 1993
Catania G, Salanitri G. Prevention of postoperative deep vein thrombosis by two different heparin types. International Journal of Clinical Pharmacology, Therapy and Toxicology 26: 304–309, 1988
Catania G, Salanitri T. Heparin therapy of post-phlebitic syndrome: Results of a single-blind, clinical trial of pamaparin vs calcium heparin. Stampa Medica Europea 13: 17–27, 1993
Chiapuzzo E, Orengo GB, Ottria G, Chiapuzzo A, Palazzini E, et al. The use of low molecular weight heparins for postsurgical deep venous thrombosis prevention in orthopaedic patients. Journal of International Medical Research 16: 359–366, 1988
Ciuffetti G, Mannarino E, Pasqualini L, Mercuri M, Lennie SE, et al. The haemorheological role of cellular factors in peripheral vascular disease. Vasa-Journal of Vascular Diseases 17: 168–170, 1988
Collins R, Scrimgeour A, Yusuf S, Peto R. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomised trials in general, orthopaedic and urologic surgery. New England Journal of Medicine 318: 1162–1173, 1988
Corrado F, Fini M, Severini G, Benati A, Monetti N, et al. Low molecular weight heparin (Fluxum) prevention of post-operative thrombosis in urological surgery: a controlled study. Clinical Trials Journal 26: 138–148, 1989
Cortellini P, Peletti F, Simonazzi M, Palazzini E, Fusillo M. Prevention of post-operative thromboembolism: a clinical trial in urological patients treated with a new low molecular weight heparin. Acta Therapeutica 14: 135–144, 1988
Cziraky MJ, Spinier SA. Low-molecular weight heparins for the treatment of deep-vein thrombosis. Clinical Pharmacy 12: 892–899, 1993
Della Marchina M, Renzi G, Renzi C, Palazzini E. Low molecular weight heparins in chronic venous insufficiency: controlled trial of pamaparin efficacy and tolerability. British Journal of Clinical Research 4: 29–36, 1993
Dettori AB, Babbini M. Human Pharmacology of a low-molecular-weight heparin (Alfa-LMWH): an update. Medicinal Research Reviews 12: 373–389, 1992
Dettori AG, Tagliaferri A, Dall’ aglio E, Pini M. Clinical pharmacology of a new low molecular weight heparin (Alfa LMWH-Fluxum). International Angiology 7 (Suppl. 3): 7–18, 1988
Di Stefano, Giglio A, Vinci M, Romano M, Salanitri G, et al. Low molecular weight heparins for long-term therapy of peripheral vascular disease. Results of a controlled study. Current Therapeutic Research 44: 1–10, 1988
Fantuz M, Milani R, Andriuoli G, Mastacchi R, Barbanti M. Antithrombotic effect of OP/LMWH by subcutaneous route in rats. Archives Internationales de Pharmacodynarnie et de Therapie 282: 328–334, 1986
Fareed J, Walenga JM, Hoppensteadt D, Huan X, Racanelli A. Comparative study on the in vitro and in vivo activities of seven lowmolecular weight heparins. Haemostasis 18: 3–15, 1988a
Fareed J, Walenga JM, Hoppensteadt D, Racanelli A, Coyne E. Chemical and biological heterogeneity in low molecular weight heparins: implications for clinical use and standardisation. Seminars in Thrombosis and Haemostasis 15: 440–463, 1989
Fareed J, Walenga JM, Racanelli A, Hoppensteadt D, Huan X, et al. Validity of the newly established low-molecular-weight heparin standard in cross-referencing low-molecular-weight heparins. Haemostasis 18 (Suppl. 3): 33–47, 1988b
Garcea D, Martuzzi F, Santelmo N, Savoia M, Casertano MG, et al. Post-surgical deep vein thrombosis prevention: evaluation of the risk/benefit ratio of fractionated and unfractionated heparin. Current Medical Research and Opinion 12: 572–583, 1992
Gruttadauria G, Ferrara C, Musumeci S, Salanitri G, Palazzini E, et al. Subcutaneous low molecular weight heparin for postsurgical thromboembolism prevention. Medical Praxis 9: 383–391, 1988
Gruttadauria G, Palazzini E. Open study of the medical treatment of postphlebitic syndromes with a new low molecular weight heparin: parnaparin. Progress Reports 5: 13–20, 1993
Haust MD. Complications of atherosclerosis in the human abdominal aorta. Monographs of Atherosclerosis 14: 43–48, 1986
Heinrich J, Balleisen L, Schulte H, Assmann G, van de Ioo J, et al. Fibrinogen and factor VII in the prediction of coronary risk: results from the PROCAM study in healthy men. Arteriosclerosis and Thrombosis 14: 54–59, 1994
Hemker HC. A standard for low molecular weight heparin? Haemostasis 1: 1–4, 1989
Hirsh J. Rationale for development oflow-molecular-weight heparins and their clinical potential in the prevention of postoperative venous thrombosis. American Journal of Surgery 161: 512–518, 1991
Hirsh J, Levine MN. Low molecular weight heparin. Blood 79: 1–17, 1992
Hoppensteadt D, Walenga JM, Fareed J. Low molecular weight heparins: an objective overview. Drugs & Aging 2: 406–422, 1992
Kandrotas R. Heparin pharmacokinetics and pharmacodynamics. Clinical Pharmacokinetics 22: 359–374, 1992
Kannel WB, Wolf PA, Castelli WP, D’ Agostino. Fibrinogen and the risk of cardiovascular disease: the Framingham study. Journal of the American Medical Association 258: 1183–1186, 1987
Kikta MJ, Keller MP, Humphrey PW, Silver D. Can low molecular weight heparins and heparinoids be safely given to patients with heparin-induced thrombocytopenia syndrome? Surgery 114: 705–710, 1993
Laguardia AM, Caroli GC. Prevention of deep vein thrombosis in orthopaedic surgery. Comparison of two different protocols with low molecular weight heparin (‘Fluxum’). Current Medical Research and Opinion 12: 584–593, 1992
Lecompte T, Luo SK, Stieltjes N, Lecrubier C, Samama MM. Correspondence. Lancet 338: 1217, 1991
Legnani C, Maccaferri M, Palareti G, Ludovici S, Guazzaloca G, et al. Perioperative prophylaxis with a low molecular weight heparin reduces late PAI-1 levels after gynaecological surgery. Fibrinolysis 4: 241–245, 1990
Leizorovicz A, Haugh MC, Boissel J-P. Correspondence. Lancet 340: 1102–1103, 1992
Leizorovicz A, Haugh MC, Chapuis F-R, Samama MM, Boissel J-P. Low molecular weight heparin in prevention of perioperative thrombosis. British Medical Journal 305: 913–920, 1992
Luttichau U, Palazzini E. Antithrombotic therapy in phlebopatbies of lower limbs: a controlled study oflow molecular weight heparin versus heparin calcium. European Review for Medical and Pharmacological Sciences 11: 351–358, 1989
Mangialardi N, Matteoni R, Serrao E, Gargiulo A. Medium term treatment of thrombotic pathologies of the lower limbs with a new LMW heparin. Medical Praxis 12: 1–7, 1991
Mannarino E, Pasqualini L, Innocente S, Orlandi U, Scricciolo V, et al. Efficacy of low-molecular-weight heparin in the management of intermittent claudication. Angiology 42: 1–7, 1991
Martines G, Restori G. Estimation of the dose-effect relationship of a low molecular weight heparin (Fluxum) through meta-analysis of clinical data. International Review of Medical Sciences 2: 79–90, 1990
Mascali F, Condorelli A, Salanitri G, Palazzini E, Iani P, et al. Postsurgery thromboembolism prevention by LMW heparin subcutaneous administration. European Review for Medical and Pharmacological Sciences 10: 135–141, 1988
Mastacchi R, Barbanti M, Bianchini P, Osima B. Interaction of a new low molecular weight heparin (OP/LMWH) with human platelets. Agents and Actions 17: 512–514, 1985
Melandri G, Branzi A, Semprini F, Cervi V, Magnani B. Effects of two dosages of subcutaneous low molecular weight heparin (parnaparin) and of unfractionated heparin on fibrin formation and lipolysis in acute myocardial infarction. Thrombosis Research 66: 141–150, 1992
Melandri G, Semprini F, Cervi V, Candiotti N, Branzi A, et al. Comparison of efficacy of low molecular weight heparin (parnaparin) with that of unfractionated heparin in the presence of activated platelets in healthy subjects. American Journal of Cardiology 72: 450–454, 1993a
Melandri G, Semprini F, Cervi V, Candiotti N, Palazzini E, et al. Benefit of adding low molecular weight heparin to the conventional treatment of stable angina pectoris. Circulation 88: 2517–2523, 1993b
Milani MR, Palazzini E. Neutralization of Fluxum, a low molecular weight heparin, by protamine. Progress Reports 2: 45–49, 1990
Mohr VD, Lenz J. Heparin-assoziierte thrombocytopenie, thrombose und embolie. Unerwünschte wirkung der thromboembolieprophylaxe mit dem niedermolekularen heparin enoxaparin. Chirurg 62: 686–690, 1991
Notarbartolo A, Salanitri G, Davì G, Averna M, Barbagallo C, et al. Low molecular weight heparin in the short and long-term treatment of deep vein thrombosis in diabetic subjects. Medical Praxis 9: 393–405, 1988
Nurmohamed MT, Rosendaal FR, Buller HR, Dekker E, Hommes DW, et al. Low-molecular-weight heparin versus standard heparin in general and orthopaedic surgery: a meta-analysis. Lancet 340: 152–156, 1992
Ockleford P. Heparin 1986. Indications and effective use. Drugs 31: 81–92, 1986
Ockleford PA, Carter CJ, Mitchell L, Hirsh J. Discordance between the anit-Xa activity and the antithrombotic activity of an ultra-low molecular weight heparin fraction. Thrombosis Research 28: 401–409, 1982
Palareti G, Legnani C, Bianchini B, Guazzaloca G, Maccaferri M, et al. Pharmacodynamic effects on blood coagulation of a new low molecular weight heparin in healthy volunteers and gynaecological surgery patients. International Angiology 8: 47–52, 1989
Palmieri G, Ambrosi G, Agrati AM, Ferraro G, Marcozzi S. A new low molecular weight heparin in the treatment of peripheral arterial disease. International Angiology 7 (Suppl. 3): 41–47, 1988b
Palmieri GC, Ambrosi G, Ferrero G, Palazzini E, Cossarizza A, et al. Kinetic control of low molecular weight heparin antithrombotic activity. European Review for Medical & Pharmacological Sciences 10: 187–192, 1988a
Palmieri G, Palazzini E, Cossarizza A, Ambrosi G, Ferrero G, et al. Bioavailability study on the sodium and calcium salts of a new low molecular weight heparin, given subcutaneously as a single dose. International Journal of Drugs and Therapy 5: 185–188, 1988c
Pellegrino A, Balta D, De Girolamo C, Di Ceglie F, Pappalettera F, et al. Prevention of post-surgical deep vein thrombosis in urology. Prophylactic use of a new low molecular weight heparin. Clinical Trials Journal 25: 164–171, 1988
Pini M, Tagliaferri A, Manotti C, Lasagni F, Rinaldi E, et al. Low molecular weight heparin (Alfa LHWH) compared with unfractionated heparin in prevention of deep-vein thrombosis after hip fractures. International Angiology 8: 134–139, 1989
Romeo G, Salanitri G, Catania G. Time-course of anti-Xa effects of calcium heparin and low-molecular-weight heparin given s.c: Insights for thrombosis prevention. Drugs Under Experimental and Clinical Research 14: 423–427, 1988
Salcuni PF, Azzarone M, Palazzini E. A new low molecular weight heparin for deep vein thrombosis prevention: effectiveness in post-operative patients. Current Therapeutic Research 43: 824–831, 1988
Salzman EW, Rosenberg RD, Smith MH, Lindon JN, Favreau L. Effect of heparin and heparin fractions on platelet aggregation. Journal of Clinical Investigation 65: 64–73, 1980
Simoni G, Lucertini G, Decian F. Low molecular weight heparins: therapeutic insight in peripheral arterial occlusive disease. Clinical Trials and Meta-Analysis 28: 137–145, 1993
Speziale F, Verardi S, Taurino M, Nicolini G, Rizzo L, et al. Low molecular weight heparin prevention of post-operative deep vein thrombosis in vascular surgery. Pharmatherapeutica 5: 261–268, 1988
Tartaglia P, Perolo F, D’ Ales A. Effectiveness of thrombosis prevention with a low molecular weight heparin preparation in gynaecological patients undergoing surgery: an open study. Current Medical Research and Opinion 11: 360–365, 1989
Tesi M, Bronchi GF, Carini A, Morfini M, Cinotti S, et al. Efficacy and safety of a new low molecular weight heparin in the medium-term treatment of atherosclerotic arteriopathy of the lower limbs. Journal of Drug Development 2: 73–82, 1989
Valle I, Sola G, Origone A. Controlled clinical study of the efficacy of a new low molecular weight heparin administered subcutaneously to prevent post-operative deep venous thrombosis. Current Medical Research and Opinion 11: 80–86, 1988
Verardi S, Baroni B, Ippoliti A, Tozzi A, Ramundo A. Efficacy and tolerability of a new low molecular weight heparin in the treatment of phlebopathies. NAM 7,8: 383–386, 1991
Verardi S, Casciani CU, Babbini M, Palazzini E. Comparative dynamics of the effects of two salts of a low molecular weight heparin in surgical patients. Journal of Drug Development 1: 87–91, 1988b
Verardi S, Casciani CU, Nicora E, Forzano F, Origone A, et al. A multicentre study on LMW-heparin effectiveness in preventing postsurgical thrombosis. International Angiology 7 (Suppl. 3.): 19–24, 1988c
Verardi S, Cortese F, Baroni B, Boffo V, Casciani CU, et al. Deep vein thrombosis prevention in surgical patients: effectiveness and safety of a new low-molecular-weight heparin. Current Therapeutic Research 46: 366–372, 1989
Verardi S, Ippoliti A, Palazzini E, Fusillo M. Behaviour of some coagulation parameters after high dose LMWH subcutaneous administration in healthy volunteers. Clinica & Terapia Cardiovascolare 7: 313–317, 1988a
Verardi S, Ippoliti A, Pistolese GR. Antithrombotic treatment during acute inflammatory complications of patients affected by postphlebytic syndrome: LMW-heparin versus standard heparin. International Angiology 7 (Suppl. 3): 33–40, 1988d
Verstraete M. Pharmacotherapeutic aspects of unfractionated and low molecular weight heparins. Drugs 40: 498–530, 1990
Verstraete M, Boogaerts MA. Haematological disorders. Avery’s Drug Treatment 3rd ed. pp. 591–675, Adis Press, Auckland, 1987.
Warkentin TE, Kelton JG. Heparin-induced thrombocytopenia. Progress in Thrombosis and Haemostasis 10: 1–34, 1991
Yarnell JWC, Baker IA, Sweetnam PM, Bainton D, O’Brien JR, et al. Fibrinogen, viscosity and white blood cell count are major risk factors for ischaemic heart disease. Circulation 83: 836–844, 1991
Zanghi M, Morici V, Costanzo M, Astuto L, Salanitri G. Deep vein thrombosis: new therapy by means of low molecular weight heparins. Journal of International Medical Research 16: 474–484, 1988
Zinicola N, Cerruti R. Deep vein thrombosis of the lower limbs: Results of long term treatment with a new low molecular weight heparin. Farmaci & Terapia/International Journal on Drugs and Therapy 6: 147–151, 1989
Author information
Authors and Affiliations
Additional information
Various sections of the manuscript reviewed by: J.E. Ansell, Department of Medicine, University of Massachusetts Medical Center, Worcester, Massachusetts, USA; G. Becchi, First General Surgery Division, Civil Hospitals, Sampierdarena, Genoa, Italy; A.G. Dettori, Fifth Department of General Medicine, Regional Hospital, Parma, Italy; D.A. Hoppensteadt, Haemostasis and Thrombosis Research Laboratories, Loyola University Medical Center, Chicago, Illinois, USA; K. Kario, Department of Internal Medicine, Hyogo Prefectural Awaji Hospital, Hyogo, Japan; T. Matsuo, Department of Internal Medicine, Hyogo Prefectural Awaji Hospital, Hyogo, Japan; M.T. Nurmohamed, Centre for Haemostasis, Thrombosis, Atherosclerosis and Inflammation Research, Meibergdreef, Amsterdam, The Netherlands; P. Ockelford, Diagnostic Laboratory, Auckland, New Zealand; M. Samama, Laboratoire Centrale d’Hématologie, Hôtel-Dieu, Paris, France; Y. Yui, Department of Internal Medicine, Kyoto University, Kyoto, Japan.
Rights and permissions
About this article
Cite this article
Frampton, J.E., Faulds, D. Parnaparin. Drugs 47, 652–676 (1994). https://doi.org/10.2165/00003495-199447040-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199447040-00007