Abstract
Synopsis
Fludarabine is a fluorinated purine analogue with antineoplastic activity in lymphoproliferative malignancies. Noncomparative studies have shown response rates in patients with chronic lymphocytic leukaemia (CLL) that appear to be at least equivalent to those achieved with conventional therapy. Prolymphocytic leukaemia, a form of CLL which is often resistant to conventional chemotherapy, has also responded to treatment with fludarabine. Fludarabine combined with prednisone has also induced good response rates in patients with CLL but the limited data suggest that this does not offer an advantage over fludarabine alone. Several clinical trials have demonstrated a good cytoreductive response in patients with advanced, low grade non-Hodgkin’s lymphoma, particularly that of follicular histology. Preliminary evidence indicates fludarabine in combination with cytarabine has therapeutic activity as salvage therapy in patients with acute leukaemia.
Myelosuppression has been the major dose-limiting adverse effect reported in clinical trials of fludarabine. A reduction in CD4+ cell count may be associated with the increased incidence of fever and opportunistic infections in fludarabine recipients. Nausea and vomiting have also been commonly reported, but these are generally mild to moderate in severity. Reversible neurotoxicity has also been occasionally reported.
Thus, fludarabine appears to offer a viable alternative to currently used treatments in CLL and low grade non-Hodgkin’s lymphoma. Other potential areas of use for fludarabine include acute leukaemias, Waidenstrom’s macroglobulinaemia and mycosis fungoides. However, trials of fludarabine in comparison with currently used therapies and also in combination with other antineoplastic agents are required to fully define its role in the treatment of lymphoid malignancies. In addition, because relapse in these diseases is common, long term trials assessing improvement in survival duration and quality of life would be of value.
Pharmacodynamic Properties
Fludarabine, a purine analogue, has demonstrated activity in culture and/or in murine tumour models against a number of human tumour types including non-Hodgkin’s lymphoma, leukaemia, breast cancer, non-small cell lung cancer and ovarian cancer. In patients with nonhaematological malignancies receiving fludarabine, T cell counts were decreased to a greater extent than were B cell counts.
Fludarabine monophosphate is dephosphorylated to the metabolite 9-β-arabinofuranosyl-2-fluoroadenine (F-Ara-A) within 5 minutes of intravenous infusion. F-Ara-A is then transported into the cell where it is converted to its active form, F-Ara-A-triphosphate (F-Ara-ATP).
Termination of DNA or RNA synthesis by incorporation of F-Ara-ATP into the elongating chain is thought to contribute to the mechanism of action of fludarabine. Additionally, DNA and RNA polymerase, DNA primase, DNA ligase and ribonucleotide reductase enzyme activity are inhibited by fludarabine, while deoxycytidine kinase activity is enhanced.
Fludarabine appears to enhance the activity of a number of antitumour agents in vitro. Increased cell death was noted when fludarabine was combined with gallium nitrate, cytarabine (ARA-C), mitoxantrone, or cytarabine plus cisplatin. Concentrations of cytarabine triphosphate, the cytotoxic metabolite of cytarabine, were increased by pre-exposure to fludarabine both in vitro and in vivo. Dose- and schedule-dependent radiosensitisation was also noted with fludarabine in in vivo models.
Pharmacokinetic Properties
Following intravenous administration fludarabine is rapidly dephosphorylated to F-Ara-A; peak concentrations of F-Ara-A were observed at the first sampling time in all patients evaluated and it is the pharmacokinetic profile of this compound that has been determined.
Volumes of distribution (44.2 to 96.2 vs 10.8 L/m2) and plasma clearances (4.1 and 9.1 vs 0.7 L/h/m2) calculated in adults were much larger than respective values determined in children. Differences in pharmacokinetic handling of the drug between adults and children, and use of different dosage regimens and/or methods to determine pharmacokinetic parameters are possible explanations for this discrepancy.
Elimination kinetics have most often been described as biphasic with a distribution phase half-life of 0.9 to 1.7 hours and an elimination phase half-life of 6.9 to 12.4 hours. Using a fluorescence assay with a sensitivity limit lower than the more commonly used ultraviolet detection method, a terminal elimination half-life of 33.5 hours was noted. In 2 studies, an initial rapid distribution phase with a half-life of 5 and 9 minutes, respectively, was also found.
A statistically significant relationship has been found between renal function parameters and the total body clearance of fludarabine, indicating a major role for renal mechanisms in its elimination. Increased serum creatinine and blood urea nitrogen levels have both been significantly correlated with decreased F-Ara-A elimination. Fludarabine-associated neutropenia appears to be more severe in patients with creatinine clearance <3 L/h (50 ml/min).
Preliminary testing of an oral form of the drug indicates bioavailability of about 70%. F-Ara-A reached peak plasma concentrations following an oral dose (unspecified) in approximately 1.5 hours.
Therapeutic Efficacy
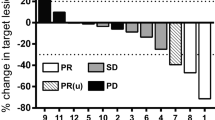
Noncomparative studies have demonstrated objective response rates of 12 to 57% in patients with chronic lymphocytic leukaemia (CLL) receiving fludarabine as salvage therapy, and up to 86% in previously untreated patients. These are at least as high as those historically noted with conventional treatments. Disease subtypes such as prolymphocytic leukaemia and the prolymphocytoid variant of CLL are often resistant to first-line therapy, but have responded to fludarabine. The combination of fludarabine with prednisone also induced a good cytoreductive response in CLL. However, the response rates do not appear to be any better than those achieved with fludarabine monotherapy and well designed comparisons have yet to be performed.
Good objective response rates have also been demonstrated in advanced, low grade non-Hodgkin’s lymphoma (NHL). Fludarabine appears to be particularly active against follicular lymphomas. Promising results have also been noted in small numbers of patients with Waldenstrom’s macroglobulinaemia and mycosis fungoides. Fludarabine in combination with cytarabine has shown activity in acute leukaemias. However, the limited clinical experience in these indications does not permit estimation of response rates.
Responses in patients with CLL or NHL following treatment with fludarabine appear to be durable. Because of the high relapse rate and chronic nature of these disorders, longer term studies evaluating the effect of fludarabine on length of survival and quality of life would be of interest. Comparative trials are also needed to assess the efficacy of fludarabine versus more established therapies. Furthermore, the use of fludarabine in combination with other antineoplastic agents needs to be more thoroughly evaluated.
Tolerability
Reversible bone marrow suppression is the most common dose-limiting effect (59% of patients), and mild to moderate nausea/vomiting (36%), fever (60%), pain (20%), and infection (33%) are also commonly reported. The latter may be due to depletion of CD4+ cells during fludarabine therapy. CNS demyelination resulting in blindness, encephalopathy, coma, and death, has occurred in patients with acute leukaemia receiving high dose fludarabine (⩾96 mg/m2/day for 5 days). Neurological toxicity has also been noted in patients receiving currently recommended fludarabine doses. However, this was reversible in all instances, except in 1 patient who also had CNS involvement of the primary malignancy. Isolated instances of interstitial pneumonitis, autoimmune haemolytic anaemia, and tumour lysis syndrome requiring treatment have also been reported.
Dosage and Administration
The recommended dosage of fludarabine is 25 mg/m2 administered as a 30-minute intravenous infusion daily for 5 days and repeated at 28-day intervals. In the instance of sustained adverse effects, the dosage may be decreased, or treatment delayed or withdrawn. Dosage reduction should be considered in patients with creatinine clearance rates ⩽3 L/h (50 ml/min). Decreased dosage may also be necessary in elderly patients or those with a low bone marrow reserve in whom severe adverse effects are noted.
Similar content being viewed by others
References
Ajani JA, Abbruzzese JL, Faintuch JS, Blackburn R, Levin B, et al. Phase II study of fludarabine phosphate in patients with advanced colorectal carcinoma. Investigational New Drugs 6: 47–50, 1988
Anaissie E, Kontoyiannis DP, Kantarjian H, Elting L, Robertson LE, et al. Listerosis in patients with chronic lymphocytic leukemia who were treated with fludarabine and prednisone. Annals of Internal Medicine 117: 466–469, 1992
Avramis VI. Pharmacodynamics and proposed mechanism of therapeutic action and host toxicity of 9-β-D-arabinofuranosyl-2-fluoroadenine monophosphate (F-ara-AMP) in P388 murine leukaemia-bearing mice. Cancer Investigation 7: 129–137, 1989
Avramis VI, Champagne J, Sato J, Krailo M, Ettinger LJ, et al. Pharmacology of fludarabine phosphate after a phase I/II trial by a loading bolus and continuous infusion in pediatric patients. Cancer Research 50: 7226–7231, 1990
Avramis VI, Plunkett W. Metabolism of 9-β-D-arabinosyl-2-fluoroadenine-5′phosphate by mice bearing P388 leukemia. Cancer Drug Delivery 1: 1–10, 1983
Balducci L, Blumenstein B, Von Hoff DD, Davis M, Hynes HE, et al. Evaluation of fludarabine phosphate in renal cell carcinoma. A Southwest Oncology Group Study. Cancer Treatment Reports 71: 543–544, 1987
Barrueco JR, Jacobsen DM, Chang C-H, Brockman RW, Sirotnak FM. Proposed mechanism of therapeutic selectivity for 9-β-D-Arabinofuranosyl-2 -fluoroadenine against murine leukemia based upon lower capacities for transport and phosphorylation in proliferative intestinal epithelium compared to tumor cells. Cancer Research 47: 700–706, 1987
Bastion Y, Coiffier B, Dumontet C, Espinouse D, Bryon PA. Severe autoimmune hemolytic anemia in two patients treated with fludarabine for chronic lymphocytic leukemia. Annals of Oncology 3: 171–173, 1992
Binet JL, Auquier A, Dighiero G, Chastang C, Piguet H, et al. A new prognostic classification of chronic lymphocytic leukemia derived from multivariate survival analysis. Cancer 48: 198–206, 1981
Boldt DH, Von Hoff DD, Kuhn JG, Hersh M. Effects on human peripheral lymphocytes of in vivo administration of 9-β-D-ar-abinofuranosyl-2 -fluoroadenine-5’-monophosphate (NSC 312887), a new purine antimetabolite. Cancer Research 44: 4661–4666, 1984
Brockman RW, Cheng Y-C, Schabel Jr FM, Montgomerey JA. Metabolism and chemotherapeutic activity of 9-β-D-arabinofuranosyl-2-fluoroadenine against murine leukemia L1210 and evidence for its phosphorylation by deoxycytidine kinase. Cancer Research 40: 3610–3615, 1980
Brockman RW, Schabel Jr FM, Montgomery JA. Biologic activity of 9-β-D-arabinofuranosyl-2-fluoroadenine, a metabolically stable analog of 9-β-D-arabinofuranosyladenine. Biochemical Pharmacology 26: 2193–2196, 1977
Carpenter Jr, JT, Vogel CL, Wang G, Raney M. Phase II evaluation of fludarabine in patients with metastatic breast cancer. A Southeastern Cancer Study Group Trial. Cancer Treatment Reports 70: 1235–1236, 1986
Cascino TL, Brown LD, Morton RF, Everson LK, Marschke RF, et al. Evaluation of fludarabine phosphate in patients with recurrent glioma. American Journal of Clinical Oncology 11: 586–588, 1988
Catapano CV, Qiu J, Perrino FW, Fernandes DJ. Primer RNA chain termination induced by fludarabine phosphate. Proceedings of the American Association for Cancer Research 33: 542, 19–1992
Catapano CV, Chandler KB, Fernandes DJ. Inhibition of primer RNA formation in CCRF-CEM leukemia cells by fludarabine triphosphate. Cancer Research 51: 1829–1835, 1991
Cervantes F, Salgado C, Montserrat E, Rozman C. Fludarabine for prolymphocytic leucaemia and risk of interstitial pneumonitis. Lancet 336: 1130, 1990
Champlin R, Golde DW. The leukemias. In Wilson et al. (Eds) Harrisons principles of internal medicine, pp. 1552–1561, 12th ed, McGraw-Hill Inc., New York, 1991
Cheson BD; New chemotherapeutic agents for non-Hodgkin’s lymphomas. Hematology/Oncology Clinics of North America 5: 1027–1050, 1991
Cheson BD. New therapeutic advances in chronic lymphocytic leukemia. Drugs of Today 26: 279–287, 1990
Cheson BD, Bennett JM, Rai KR, Grever MR, Kay NE. Guidelines for clinical protocols for chronic lymphocytic leukemia: Recommendations of the National Cancer Institute-Sponsored Working Group. American Journal of Hematology 29: 152–163, 1988
Chun HG, Leyland-Jones BR, Caryk SM, Hoth DF. Central nervous system toxicity of fludarabine phosphate. Cancer Treatment Reports 70: 1225–1228, 1986
Chun HG, Leyland-Jones B, Cheson BD. Fludarabine phosphate: a synthetic purine antimetabolite with significant activity against lymphoid malignancies. Journal of Clinical Oncology 9: 175–188, 1991
Cohen A, Barankiewicz J, Lederman HM, Gelfand EW. Purine and pyrimidine metabolism in human T lymphocytes. Journal of Biological Chemistry 258: 12334–12340, 1983
Cohen RB, Abdallah JM, Gray JR, Foss F. Reversible neurologic toxicity in patients treated with standard-dose fludarabine phosphate for mycosis fungoides and chronic lymphocytic leukemia. Annals of Internal Medicine 118: 114–116, 1993
Danhauser L, Plunkett W, Liliemark J, Gandhi V, Iacoboni S, et al. Comparison between the plasma and intracellular pharmacology of 1-β-D-arabinofuranosylcytosine and 9-β-D-arabinofuranosyl-2-fluoroadenine 5’-monophosphate in patients with relapsed leukemia. Leukemia 1: 638–643, 1987
Danhauser L, Plunkett W, Keating M, Cabanillas F. 9-β-D-arabinofuranosyl -2-fluoroadenine-5’-monophosphate pharmacokinetics in plasma and tumor cells of patients with relapsed leukemia land lymphoma. Cancer Chemotherapy and Pharmacology 18: 145–152, 1986
Dow LW, Bell DE, Poulakos L, Fridland A. Differences in metabolism and cytotoxicity between 9-β-D arabinofuranosyladenine and 9-β-D -arabinofuranosyl-2-fluoradenine in human leukemic lymphoblasts. Cancer Research 40: 1405–1410, 1980
Elias L, Stock-Novak D, Grever M, Weick J, Chapman R, et al. A phase I trial of combination fludarabine and chlorambucil in chronic lymphocytic leukemia. Abstract 745. Proceedings of the American Society of Clinical Oncology 10: 221, 1991
Estey E, Kantarjian H, Plunkett W, Gandhi V, Rios M, et al. Treatment of refractory acute myelogenous leukemia with fludarabine + cytosine arabinoside. Abstract 884. Proceedings of the American Society of Clinical Oncology 11: 268, 1992
Fenchel K, Bergmann L, Jahn B, Mitrou P, Hoelzer D. Fludarabine phosphate in refractory chronic lymphocytic leukemia. Onkologie 14 (Suppl. 2): 190, 1991
Foss F, Ihde D, Phelps R, Fischmann A, Schechter G, et al. Phase II study of fludarabine and interferon-alfa-2a in advanced mycosis fungoides/Sezary syndrome (MF/SS). Abstract 1065. Proceedings of the American Society of Clinical Oncology 11: 315, 1992
Gandhi V, Estey E, Keating MJ, Plunkett W. Fludarabine potentiates metabolism of cytarabine in patients with acute myelogenous leukemia during therapy. Journal of Clinical Oncology 11: 116–124, 1993
Gandhi V, Kemena A, Keating MJ, Plunkett W. Fludarabine infusion potentiates arabinosylcytosine metabolism in lymphocytes of patients with chronic lymphocytic leukemia. Cancer Research 52: 897–903, 1992
Gandhi V, Nowak B, Keating MJ, Plunkett W. Modulation of arabinosylcytosine metabolism by arabinosyl-2-fluoroadenine in lymphocytes from patients with chronic lymphocytic leukemia: implications for combination therapy. Blood 74: 2070–2075, 1989
Gandhi V, Plunkett W. Modulation of arabinosylnucleoside metabolism by arabinosylnucleotides in human leukemia cells. Cancer Research 48: 329–334, 1988
Gregoire V, Hunter N, Milas L, Brock WA, Plunkett WK. Enhancement of radiation response by fludarabine in murine tumor models. Proceedings of the American Association for Cancer Research 33: 85, 1992
Grever MR, Kopecky KJ, Coltman CA, Files JC, Greenberg BR et al. Fludarabine monophosphate: a potentially useful agent in chronic lymphocytic leukemia. Nouvelle Revue Francaise d’Hematologie 30: 457–459, 1988
Grever M, Leiby J, Kraut E, Metz E, Neidhart J, et al. A comprehensive phase I and II clinical investigation of fludarabine phosphate. Seminars in Oncology 17 (Suppl. 8): 39–48, 1990
Harvey WH, Fleming TR, Beitran G, Saiers JH, Oishi N, et al. Phase II study of fludarabine phosphate in previously untreated patients with hepatoma. A Southwest Oncology Group Study. Cancer Treatment Reports 71: 1111–1112, 1987a
Harvey WH, Fleming TR, Von Hoff DD, Katterhagen JG, Coltman Jr CA. Phase II study of fludarabine phosphate in previously untreated patients with colorectal carcinoma. A Southwest Oncology Group Study. Cancer Treatment Reports 71: 1319–1320, 1987b
Hersh MR, Kuhn JG, Phillips JL, Clark G, Ludden TM, et al. Pharmacokinetic study of fludarabine phosphate (NSC 312887). Cancer Chemotherapy and Pharmacology 17: 277–280, 1986
Hiddeman W, Rottman R, Wörmann, Theil A, Essink M, et al. Treatment of advanced chronic lymphocytic leukemia by fludarabine: Results of a clinical phase-II study. Annals of Hematology 63: 1–4, 1991
Hochster HS, Kim K, Green MD, Mann RB, Neiman RS et al. Activity of fludarabine in previously treated non-Hodgkin’s lowgrade lymphoma. Results of an Eastern Cooperative Oncology Group Study. Journal of Clinical Oncology 10: 28–32, 1992
Huang P, Chubb S, Plunkett W. Termination of DNA synthesis by 9-β-D-arabinofuranosyl-2-fluoroadenine. A mechanism for cytotoxicity. Journal of Biological Chemistry 265: 16617–16625, 1990
Huang P, Plunkett W. Action of 9-β-D-arabinofuranosyl-2-fluoroadenine on RNA metabolism. Molecular Pharmacology 39: 449–455, 1991
Hurst PG, Habib MP, Garewal H, Bluestein M, Paquin M, et al. Pulmonary toxicity associated with fludarabine monophosphate. Investigational New Drugs 5: 207–210, 1987
International Workshop on Chronic Lymphocytic Leukemia. Chronic lymphocytic leukemia: recommendations for diagnosis, staging, and response criteria. Annals of Internal Medicine 110: 236–238, 1989
Johnson SA, Richardson D, Hpokins J, Howe D, Phillips MJ. Complete remission after fludarabine for chronic lymphocytic leukemia. Correspondence. Blood 81: 560–566, 1993
Kane GC, McMichael AJ, Patrick H, Erslev AJ. Pulmonary toxicity and acute respiratory failure associated with fludarabine monophosphate. Respiratory Medicine 86: 261–263, 1992
Kantarjian HM, Alexanian R, Koller CA, Kurzrock R, Keating MJ. Fludarabine therapy in macroglobuljnemic lymphoma. Blood 75: 1928–1931, 1990
Kantarjian HM, Schachner J, Keating MJ. Fludarabine therapy in hairy cell leukemia. Cancer 67: 1291–1293, 1991
Kavanagh JJ, Stringer CA, Copeland LJ, Gershenson DM, Saul P. Phase II trial of fludarabine in patients with epithelial ovarian cancer. Cancer Treatment Reports 70: 425–426, 1986
Keating MJ, Kantarjian H, O’Brien S, Koller C, Talpaz M, et al. Fludarabine: a new agent with marked cytoreductive activity in untreated chronic lymphocytic leukemia. Journal of Clinical Oncology 9: 44–49, 1991
Keating MJ, Kantarjian H, Talpaz M, Redman J, Koller C et al. Fludarabine: a new agent with major activity against chronic lymphocytic leukemia. Blood 74: 19–25, 1989
Kemena A, Fernandez M, Bauman J, Keating M, Plunkett W. A sensitive fluorescence assay for quantitation of fludarabine and metabolites in biological fluids. Clinica Chimica Acta 200: 95–106, 1991a
Kemena A, Keating M, Plunkett W. Oral bioavailability of plasma fludarabine and fludarabine triphosphate (F-ara-ATP) in peripheral CLL cells. Onkologie 14 (Suppl. 2): 83, 1991b
Kilton LJ, Benson AB, Greenberg A, Johnson P, Shapiro C et al. Phase II trial of fludarabien phosphate for adenocarcinoma of the pancreas: an Illinois Cancer Center study. Investigational New Drugs 10: 201–204, 1992
Kim JH, Alfieri AA, Kim SH, Fuks Z. The potentiation of radiation response on murine tumor by fludarabine phosphate. Cancer Letters 31: 69–76, 1986
Kish JA, Kopecky K, Samson MK, Von Hoff DD, Fletcher WS, et al. Evaluation of fludarabine phosphate in malignant melanoma. A Southwest Oncology Group study. Investigational New Drugs 9: 105–108, 1991
Knauf WU, Kreuser E-D, Pottgieβer E, Thiel E. In vitro and in vivo effectiveness of fludarabine in B-cell chronic lymphocytic leukemia (B-CLL). Abstract 203. Annals of Hematology 65 (Suppl.): A80, 1992
Koeller J, Murphy GP. Malignant lymphomas. In DiPiro JT et al. (Eds), Pharmacotherapy. A Pathophysiologic Approach, pp. 1395–1413, Elsevier, Amsterdam, 1988
Kraut EH, Chun HG. Fludarabine phosphate in refractory hairy cell leukemia. American Journal of Hematology 37: 59–60, 1991
Kraut EH, Crowley JJ, Grever MR, Keppen MD, Bonnet JD. Phase II study of fludarabine phosphate in multiple myeloma. A Southwest Oncology Group Study. Investigational New Drugs 8: 199–200, 1990
Lathan B, Diehl V, Clark GM, Von Hoff DD. Cytotoxic activity of 9-β-D-arabinofuranosyl-2-fluoroadenine 5-monophosphate (fludarabine NSC 312887) in a human tumor cloning system. European Journal of Cancer and Clinical Oncology 24: 1891–1895, 1988
Lehninger AL. Principles of biochemistry. Chapter 28. Replication and transcription of DNA, 849, 1982
Leiby JM, Snider KM, Kraut EH, Metz EN, Malspeis L. Phase II trial of 9-β-D-arabinofuranosyl-2-fluoroadenine 5’-monophosphate in non-Hodgkin’s lymphoma: prospective comparison of response with deoxycytidine kinase activity. Cancer Research 47: 2719–2722, 1987
Lichtman SM, Mittelman A, Budman DR, Puccio CA, Chun HG, et al. Phase II trial of fludarabine phosphate in multiple myeloma using a loading dose and continuous infusion schedule. Leukemia and Lymphoma 6: 61–63, 1991
List AF, Kummet TD, Adams JD, Chun HG. Tumor lysis syndrome complicating treatment of chronic lymphocytic leukemia with fludarabine phosphate. American Journal of Medicine 89: 388–390, 1990
Lundberg JH, Chitambar CR. Interaction of gallium nitrate with fludarabine and iron chelators: effects on the proliferation of human leukemic HL60 cells. Cancer Research 50: 6466–6470, 1990
Malspeis L, Grever MR, Staubus AE, Young D. Pharmacokinetics of 2-F-ara-A (9-β-D-arabinofuranosyl-2-fluoroadenine) in cancer patients during the phase I clinical investigation of fludarabine phosphate. Seminars in Oncology 17 (Suppl. 8): 18–32, 1990
Marlton P, McCarthy C, Taylor K. Letter to the editor: fludarabine-induced cytogenic remission in prolymphocytic leukemia. American Journal of Hematology 40: 71–72, 1992
Mittelman A, Ashikari R, Ahmed T, Savona S, Arnold P, et al. Phase II trial of fludarabine phosphate (F-Ara-AMP) in patients with advanced breast cancer. Cancer Chemotherapy and Pharmacology 22: 63–64, 1988
Mittelman A, Savona S, Puccio C, Chun H, Ahmed T, et al. Phase II trial of fludarabine phosphate (F-Ara-AMP) in patients with advanced head and neck cancer. Investigational New Drugs 8: S65–S67, 1990
Nadler LM. The malignant lymphomas. In Wilson et al. (Eds) Harrison’s principles of internal medicine, 12th ed. pp. 1599–1612, McGraw-Hill Inc., New York, 1991
Noker PE, Duncan GF, El Dareer SM, Hill DL. Disposition of 9-β-D-arabinofuranosyl-2-fluoroadenine 5’-phosphate in mice and dogs. Cancer Treatment Reports 67: 445, 1983
O’Brien S, Kantarjian H, Koller C, Robertson L, Bèran M, et al. Fludarabine — prednisone: a highly effective regimen in chronic lymphoeytic leukemia (CLL). Abstract 850. Proceedings of the American Society of Clinical Oncology 11: 260, 1992
Pazdur R, Samson MK, Baker LH. Fludarabine phosphate. Phase II evaluation in advanced soft-tissue sarcomas. American Journal of Clinical Oncology 10: 341–343, 1987
Plunkett W, Huang P, Gandhi V. Metabolism and action of fludarabine phosphate. Seminars in Oncology 17 (Suppl. 8): 3–17, 1990
Puccio CA, Mittelman A, Lichtman SM, Silver RT et al. A loading dose/continuous infusion schedule of fludarabine phophate in chronic lymphocytic leukemia. Journal of Clinical Oncology 9: 1562–1569, 1991
Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, et al. Clinical staging of chronic lymphocytic leukemia. Blood 46: 219–234, 1975
Raincy JM, Hill JB, Crowley J. Evaluation of fludarabine phosphate in small cell carcinoma. A Southwest Oncology Group Study. Investigational New Drugs 6: 45–46, 1988
Redman JR, Cabanillas F, Velasquez WS, McLaughlin P, Hagemeister FB. Phase II trial of fludarabine phosphate in lymphoma: an effective new agent in low-grade lymphoma. Journal of Clinical Oncology 10: 790–794, 1992
Reis Borges R, Zylberait D, Broquie G, Veyssier P. Pneumocystose, kératite herpétique, méningite à cryptocoques chez un patient VIH négatif atteint d’une LLC et traité par fludarabine. Revue de Medicine Interne 13: S 436, 1992
Robertson LE, Huh YO, Butler JJ, Pugh WC, Hirsch-Ginsberg C, et al. Response assessment in chronic lymphocytic leukemia after fludarabine plus prednisone: clinical, pathologic, immunophenotypic, and molecular analysis. Blood 80: 29–36, 1992
Sanders C, Perez EA, Lawrence HJ. Opportunistic infections in patients with chronic lymphocytic leukemia following treatment with fludarabine. Correspondence. American journal of Hematology 39: 314–315, 1992
Sato JK, Wiersma S, Krallo M, Bostrom B, Shurin S, et al. Phase I clinical and pharmacodynamic study of continuous infusion (CI) fludarabine followed by continuous infusion (CI) cytosine arabinoside (ara-C) in relpsed leukemia. Abstract 1266. Proceedings of the American Association for Cancer Research 33: 211, 1992
Shevrin DH, Lad TE, Kilton LJ, Cobleigh MA, Blough RR. Phase II trial of fludarabine phosphate in advanced renal cell carcinoma. An Illinois Cancer Council Study. Investigational New Drugs 7: 251–253, 1989
Smith OP, Mehta AB. Fludarabine monophosphate for prolymphocytic leukemia. Lancet 336: 820, 1990
Sporn JR. Sustained response of refractory prolymphocytic leukemia to fludarabine. Acta Haematologie 85: 209–211, 1991
Spriggs D, Robbins G, Mitchell T, Kufe D. Incorporation of 9-β-D-arabinofluranosyl-2-fluoroadenine into HL-60 cellular RNA and DNA. Biochemical Pharmacology 35: 247–252, 1986
Stewart CF. Chronic leukemia. In DiPiro JT et al. (Eds), Pharmacotherapy. A pathophysiologic approach, pp. 1462–1472, Elsevier, Amsterdam, 1988
Taylor SA, Crowley J, Vogel FS, Townsend JJ, Eyre HJ, et al. Phase II evaluation of fludarabine phosphate in patients with central nervous system tumors. Investigational New Drugs 9: 195–197, 1991
Tosti S, Caruso R, D’Adamo F, Picardi A, Ali Ege M, et al. Severe autoimmune hemolytic anemia in a patient with chronic lymphocytic leukemia responsive to fludarabine-based treatment. Annals of Hematology 65: 238–239, 1992
Tseng W-C, Derse D, Cheng Y-C, Brockman RW, Bennet Jr LL. In vitro biological activity of 9-β-D-arabinofuranosyl-2-fluoroadenine and the biochemical actions of its triphosphate on DNA polymerases and ribonucleotide reductase from HeLa cells. Molecular Pharmacology 21: 474–477, 1982
Von Hoff DD, Dahlberg S, Hartstock RJ, Eyre HJ. Activity of fludarabine monophosphate in patients with advanced mycosis fungoides. A Southwest Oncology Group Study. Brief Communications 82: 13, 1990
Von Hoff DD, Green S, Alberts DS, Stock-Novack DL, Surwit EA. Phase II study of fludarabine phosphate (NSC-312887) in patients with advanced endometrial cancer. American Journal of Clinical Oncology 14: 193–194, 1991
Von Hoff DD, Green S, Surwit EA, Hannigan EV, Alberts DS. Phase II study of fludarabine phosphate (NSC 312887) in patients with advanced cervical cancer. A Southwest Oncology
Group Study. American Journal of Clinical Oncology 13: 433–435, 1990
Von Hoff DD, Kronmal R, O’Toole RV, Surwit EA, Hutton JJ, et al. Phase II study of fludarabine phosphate (NSC-312887) in patients with advanced ovarian cancer. A Southwest Oncology Group Study. American Journal of Clinical Oncology 11: 146–148, 1988
Warrell Jr RP, Berman E. Phase I and II study of fludarabine phosphate in leukemia: therapeutic efficacy with delayed central nervous system toxicity. Journal of Clinical Oncology 4: 74–79, 1986
Weiss GR, Crowley J, Von Hoff DD, Taylor SA, Belt RJ, et al. Phase II study of fludarabine phosphate for the treatment of advanced non-small cell carcinoma of the lung. A Southwest Oncology Group Study. Cancer Treatment Reports 70: 1123, 1986
Weiss GB, Metch B, Von Hoff DD, Taylor SA, Saiers JH. Phase II trial of fludarabine phosphate in patients with head and neck cancer: A Southwest Oncology Group Study 71: 1313–1314, 1987
Weiss M, Kempin S, Berman E, Eardley A, Gee T, et al. Results of a phase I study of fludarabine phosphate (FAMP) plus chlorambucil (CLB) in patients with chronic lymphocytic leukemia (CLL). Abstract 914. Proceedings of the American Society of Clinical Oncology 11: 276, 1992
Whelan JS, Davis CL, Rule S, Ranson M, Smith OP, et al. Fludarabine phosphate for the treatment of low grade lymphoid malignancy. British Journal of Cancer 64: 120–123, 1991
Whelan JS, Davis CL, Johnson SAN, Norton AJ, Rohatiner AZS, et al. Fludarabine in chronic lymphocytic leukaemia (CLL) and non-Hodgkin’s lymphoma (NHL). British Journal of Cancer 62: 499, 1990
Wijermans et al. Proceedings of the American Society of Hematology. Abstract 174, 1992
Yang LY, Trujillo JM, Keating MJ, Plunkett W. Effect of fludarabine on the tumoricidal synergy produced by the combination of arabinosylcytosine and cisplatin in human LoVo colon carcinoma cells. Proceedings of the American Association for Cancer Research 33: 443, 1992a
Yang S-N, Huang P, Plunkett W, Becher F, Chan JYH. Dual mode of inhibition of purified DNA ligase from human cells by 9-β-arabinofuranosyl-2-fluoroadenine triphosphate. Journal of Biological Chemistry 267: 2345–2349, 1992b
Author information
Authors and Affiliations
Additional information
Various sections of the manuscript reviewed by: V.I. Avramis, Division of Hematology-Oncology, Children’s Hospital, Los Angeles, California, USA; D. Catovsky, Academic Haematology and Cytogenetics, Royal Marsden Hospital, London, England; B.D. Cheson, Clinical Investigations Branch, National Cancer Institute, Bethesda, Maryland, USA; G. Ehninger, Departments of Hematology and Oncology, Medizinische Universitätsklinik and Institut für Organische Chemie der Universität Tübingen, Tübingen, Federal Republic of Germany; A.V. Hoffbrandy Department of Haematology, Gray’s Inn Division, Royal Free Hospital, London, England; G.M. Jeffery, Oncology and Haematology Unit, Dunedin Hospital, Dunedin, New Zealand; G. Juliusson, Division of Clinical Hematology and Oncology, Department of Medicine, Karolinska Institute, Huddinge, Sweden; M. Keating, Departments of Medicine and Hematology, University of Texas MD Anderson Cancer Center, Houston, Texas, USA; W.U. Knauf, Department of Hematology and Oncology Klinikum Steglitz, Free University of Berlin, Berlin, Federal Republic of Germany; J.G. Kuhn, College of Pharmacy, Departments of Medicine and Pharmacology, Clinical Pharmacy Programs at San Antonio, University of Texas, San Antonio, Texas, USA; B. Lathan, First Department of Medicine, University of Cologne, Cologne, Federal Republic of Germany.
Rights and permissions
About this article
Cite this article
Ross, S.R., McTavish, D. & Faulds, D. Fludarabine. Drugs 45, 737–759 (1993). https://doi.org/10.2165/00003495-199345050-00009
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199345050-00009