Abstract
Several investigators have demonstrated that diabetes is associated with autonomic and myocardial dysfunction. Exercise training is an efficient non-pharmacological treatment for cardiac and metabolic diseases. The aim of the present study was to investigate the effects of exercise training on hemodynamic and autonomic diabetic dysfunction. After 1 week of diabetes induction (streptozotocin, 50 mg/kg, iv), male Wistar rats (222 ± 5 g, N = 18) were submitted to exercise training for 10 weeks on a treadmill. Arterial pressure signals were obtained and processed with a data acquisition system. Autonomic function and intrinsic heart rate were studied by injecting methylatropine and propranolol. Left ventricular function was assessed in hearts perfused in vitro by the Langendorff technique. Diabetes (D) bradycardia and hypotension (D: 279 ± 9 bpm and 91 ± 4 mmHg vs 315 ± 11 bpm and 111 ± 4 mmHg in controls, C) were attenuated by training (TD: 305 ± 7 bpm and 100 ± 4 mmHg). Vagal tonus was decreased in the diabetic groups and sympathetic tonus was similar in all animals. Intrinsic heart rate was lower in D (284 ± 11 bpm) compared to C and TD (390 ± 8 and 342 ± 14 bpm, respectively). Peak systolic pressure developed at different pressures was similar for all groups, but +dP/dt max was decreased and -dP/dt max was increased in D. In conclusion, exercise training reversed hypotension and bradycardia and improved myocardial function in diabetic rats. These changes represent an adaptive response to the demands of training, supporting a positive role of physical activity in the management of diabetes.
experimental diabetes; exercise training; arterial pressure; autonomic control; myocardial contractility
Braz J Med Biol Res, June 2000, Volume 33(6) 635-641
Effects of exercise training on autonomic and myocardial dysfunction in streptozotocin-diabetic rats
K.L.D. De Angelis1, A.R. Oliveira1,2, P. Dall'Ago1, L.R.A. Peixoto1, G. Gadonski1, S. Lacchini1, T.G. Fernandes1 and M.C. Irigoyen1,3
1Laboratório de Fisiologia Cardiovascular, Departamento de Fisiologia, Instituto de Ciências Básicas da Saúde, and 2Laboratório de Pesquisa do Exercício, Faculdade de Educação Física, Universidade Federal do Rio Grande do Sul, RS, Brasil
3Unidade de Hipertensão e Divisão de Experimentação, Instituto do Coração (Incor), Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo, São Paulo, SP, Brasil
References
Correspondence and Footnotes Correspondence and Footnotes

Several investigators have demonstrated that diabetes is associated with autonomic and myocardial dysfunction. Exercise training is an efficient non-pharmacological treatment for cardiac and metabolic diseases. The aim of the present study was to investigate the effects of exercise training on hemodynamic and autonomic diabetic dysfunction. After 1 week of diabetes induction (streptozotocin, 50 mg/kg, iv), male Wistar rats (222 ± 5 g, N = 18) were submitted to exercise training for 10 weeks on a treadmill. Arterial pressure signals were obtained and processed with a data acquisition system. Autonomic function and intrinsic heart rate were studied by injecting methylatropine and propranolol. Left ventricular function was assessed in hearts perfused in vitro by the Langendorff technique. Diabetes (D) bradycardia and hypotension (D: 279 ± 9 bpm and 91 ± 4 mmHg vs 315 ± 11 bpm and 111 ± 4 mmHg in controls, C) were attenuated by training (TD: 305 ± 7 bpm and 100 ± 4 mmHg). Vagal tonus was decreased in the diabetic groups and sympathetic tonus was similar in all animals. Intrinsic heart rate was lower in D (284 ± 11 bpm) compared to C and TD (390 ± 8 and 342 ± 14 bpm, respectively). Peak systolic pressure developed at different pressures was similar for all groups, but +dP/dt max was decreased and -dP/dt max was increased in D. In conclusion, exercise training reversed hypotension and bradycardia and improved myocardial function in diabetic rats. These changes represent an adaptive response to the demands of training, supporting a positive role of physical activity in the management of diabetes.
Key words: experimental diabetes, exercise training, arterial pressure, autonomic control, myocardial contractility
Abstract
Introduction
Diabetes is a chronic metabolic disorder associated with secondary complications in the cardiovascular system and autonomic control in humans and animals (1-4). Higher morbidity and mortality have been observed in symptomatic patients with diabetes mellitus and with autonomic neuropathy (5). In rats, degenerative changes in autonomic neurons were observed from 3 days to several weeks after streptozotocin (STZ) injection (6,7). In our laboratory, we observed that 5 days after diabetes induction with STZ, rats presented lower arterial pressure (AP) and heart rate (HR), as well as a reduction in intrinsic HR (IHR) and in vagal tonus, suggesting early autonomic dysfunction (2,8). Those changes in AP, HR and autonomic function, evaluated by AP variability, were reversible by chronic insulin treatment (3). Studies on animals and humans have shown that diabetic subjects exhibit depressed myocardial contractile performance, particularly under stress conditions such as enhanced workload (1,4).
In our laboratory, exercise training applied to young and aged normotensive and young hypertensive rats improved cardiovascular and autonomic control (9,10). Moreover, we demonstrated that physical activity enhanced the peripheral action of insulin in aged normotensive and hypertensive rats (9,11). Exercise training has also been shown to limit the depression in myocardial pump function in diabetic subjects (12-14). However, there are few data in the literature about the effects of exercise on autonomic and hemodynamic function in experimental diabetes. In the present study, we evaluated changes induced by exercise training in AP, HR and autonomic control, as well as in myocardial function in STZ-diabetic rats.
Material and Methods
Experiments were performed on 18 male Wistar rats (222 ± 5 g) from the Animal House of Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil, receiving standard laboratory chow and water ad libitum. The animals were housed in individual cages in a temperature-controlled room (22oC) with a 12-h dark-light cycle. All surgical procedures and protocols used were in accordance with the Guidelines for Ethical Care of Experimental Animals and were approved by the International Animal Care and Use Committee.
The rats were randomly assigned to one of three groups: sedentary control (C, N = 5), sedentary diabetic (D, N = 6) or trained diabetic (TD, N = 7). Animals were made diabetic by a single injection of STZ (50 mg/kg, iv; Sigma Chemical Co., St. Louis, MO, USA) dissolved in citrate buffer, pH 4.5. Rats were fasted overnight before STZ injection.
The animals were submitted to low-intensity exercise training (50% VO2 max) one week after diabetes induction on a treadmill twice a day (one hour each time), 5 days a week for 10 weeks, with gradually progression to a speed of 31 m/min, as described in detail elsewhere (15).
After the last training session, two catheters filled with 0.06 ml saline were implanted under ether anesthesia into the femoral artery and vein (PE-10) for direct measurements of AP and drug administration, respectively. Rats receiving food and water ad libitum were studied 1 day after catheter placement; the rats were conscious and allowed to move freely during experiments. The arterial cannula was connected to a strain-gauge transducer (P23Db, Gould-Statham, Oxnard, CA, USA), and blood pressure signals were recorded over a 20-min period by a microcomputer equipped with an analog-to-digital converter board (CODAS, 1-kHz sampling frequency, Dataq Instruments, Inc., Akron, OH, USA). The recorded data were analyzed on a beat-to-beat basis to quantify changes in mean AP and HR.
Vagal and sympathetic tonus and IHR were studied (2) by injecting methylatropine (3 mg/kg, iv; Sigma) and propranolol (4 mg/kg, iv; Sigma) in a maximal volume of 0.2 ml per injection. Resting HR was recorded while the rats were in their cages in an unrestrained state and methylatropine was injected immediately after the recording. Because the HR response to these drugs reaches its peak within 10 to 15 min (2), this time interval was allowed to elapse before HR measurement. Propranolol was injected 15 min after methylatropine, and again the response was evaluated after simultaneous blockade with propranolol and methylatropine. On the subsequent day, the sequence of injections was inverted, first propranolol and then methylatropine. IHR was evaluated after simultaneous blockade with propranolol and methylatropine. Sympathetic tonus was determined as the difference between maximum HR after methylatropine injection and IHR. Vagal tonus was obtained by the difference between the lowest HR after propranolol injection and IHR.
After the hemodynamic measurements the rats were killed by a blow to the neck. The chest was opened and the heart excised and perfused using a Langendorff system. The aorta was retroperfused with a modified Krebs solution (120 mM NaCl, 5.4 mM KCl, 1.8 mM MgCl2, 1.25 mM CaCl2, 27 mM NaHCO3, 2 mM NaH2CO3, 1.8 mM Na2SO4, and 11 mM glucose), pH 7.4. The solution was maintained at 37oC, gassed with 95% O2 and 5% CO2 and used for perfusion at a constant flow (11 ml/min). A latex balloon filled with water and connected to a pressure transducer (Isotec P23 XL - Hugo Sachs Elektroniks, March, Freiburg, Germany) was placed inside the left ventricle. The signals were processed using an analogic-digital conversion system including a microcomputer (IBM - PC 486), an analogic-digital converter board and an Isoheart software (Hugo Sachs Elektroniks). A stabilization period of 30 min was allowed to elapse for standardization of cardiac function. After stabilization, the balloon volume was adjusted to generate a diastolic pressure of 0 mmHg. The pressure was increased in steps of 10 mmHg at 5-min intervals, reaching 10, 20, 30, 40 and 50 mmHg. The variables analyzed for each diastolic pressure used were left ventricular systolic pressure, maximum rate of rise (+dP/dt max) and maximum rate of fall (-dP/dt max).
Body weight was monitored each week during the period of physical activity. Blood samples were collected at rest in the fasting state one week after diabetes induction. Plasma glucose was measured by a colorimetric enzymatic test (Enz Color, Bio Diagnostica, Piraquara, PR, Brazil).
Data are reported as means ± SEM, and two-way ANOVA was used to compare groups, followed by the Student-Newman-Keuls test. Correlation was determined by linear regression analysis.

All rats given STZ developed severe hyperglycemia (479 ± 8 for the D group and 467 ± 17 mg/dl for the TD group) compared with controls (125 ± 7 mg/dl). Body weight was reduced in both diabetic groups as compared with sedentary control (329 ± 16 g) (P<0.01). Trained diabetic rats (241 ± 11 g) showed an increase in body weight compared with sedentary diabetic rats (195 ± 15 g) (P<0.04).
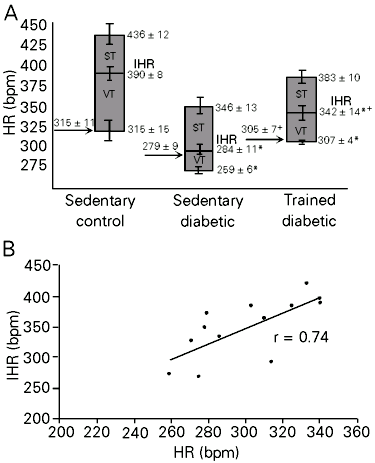
As can be seen in Table 1, exercise training induced attenuation in diabetic hypotension and bradycardia. Figure 1A shows the control HR, IHR and HR responses to drug blockade. Sedentary diabetic rats presented lower basal AP values than sedentary control animals. No differences were observed between trained diabetic rats and sedentary groups. Basal evaluations of HR showed bradycardia in the STZ sedentary group (279 ± 9 bpm) compared with the STZ trained and sedentary control groups (305 ± 7, P<0.04 and 315 ± 11 bpm, P<0.03). The IHR obtained after methylatropine and propranolol blockade was significantly lower in sedentary diabetic (284 ± 11 bpm) than in trained diabetic and control rats (342 ± 14, P<0.01 and 390 ± 8 bpm, P<0.00). A significant reduction in IHR was observed in trained diabetic rats compared to control animals (P<0.02). A positive correlation determined by linear regression was found between resting HR and IHR, showing higher HR values at higher IHR (r = 0.74, P<0.04) (Figure 1B). Vagal tonus was decreased in diabetic rats compared to sedentary control rats and sympathetic tonus was similar for all groups (Table 1).
The left ventricular function obtained for the various groups is shown in Figure 2, where pooled results of total left ventricular isovolumetric systolic pressure are plotted against left ventricular diastolic pressure. It can be seen that the increase in isovolumetric systolic pressure as a function of diastolic pressure was similar in control, diabetic and trained diabetic rats. Isolated hearts from sedentary control and trained diabetic rats developed a reduced maximum rate of rise (+dP/dt max) and an increased maximum rate of fall (-dP/dt max) compared to isolated hearts from sedentary diabetic rats (P<0.05). The overall spontaneous HR was not significantly altered by diabetes or training. HR for the three groups (bmp) was as follows: controls, 201 ± 22; diabetic rats, 170 ± 34; trained diabetic rats, 204 ± 25.
- A, Graphs showing intrinsic heart rate (IHR), sympathetic (ST) and vagal tonus (VT) in sedentary control, sedentary diabetic and trained diabetic rats. Arrows indicate basal heart rate (HR). Data are reported as means ± SEM. *P<0.05 compared to sedentary control rats; +P<0.05 compared to sedentary diabetic rats (ANOVA). B, Positive relationship between resting HR and IHR expressed by linear regression line (r = 0.74) in the diabetic groups (P<0.04).
- Influence of diastolic pressure on systolic pressure (A), maximum rate of rise (+dP/dt max) (B), and maximum rate of fall (-dP/dt max) (C) developed by the left ventricle of hearts isolated from sedentary control, sedentary diabetic and trained diabetic rats. Data are reported as means ± SEM. *P<0.05 compared to sedentary control rats; +P<0.05 compared to sedentary diabetic rats (ANOVA).
Results

The present study confirms our preliminary findings that STZ diabetes induces hypotension, bradycardia and autonomic dysfunction. Also, we observed decreased myocardial function in sedentary diabetic rats (2,3). However, the major finding of this investigation is that exercise training improves diabetes-induced dysfunction. Jackson and Carrier (16) suggested that the decrease in AP may be the result of a decreased cardiac output in diabetic sedentary rats due to hypovolemia caused by hyperglycemic osmotic diuresis. However, Cohen et al. (17) observed that these animals were polyuric with a high urine flow, reflecting the osmotic diuretic effects of glucose. The hypotension observed in the sedentary diabetic group may also have been due to an increase in parasympathetic outflow, although Maeda et al. (2) and the present data demonstrated a decrease in vagal function, suggesting that changes in AP are not related to an increase in parasympathetic outflow. Previous studies have shown that exercise training improves cardiac output in diabetic rats (12,13). Exercise also improves glucose homeostasis, reducing the glucose/insulin ratio and increasing insulin sensitivity (9,11). Moreover, exercise not only attenuates the reduction in myocardial GLUT-4 transporters (18) but also increases sarcolemmal GLUT-4 protein in diabetic rats (14). Insulin plays a critical role in this process since GLUT-4 depression and hemodynamic changes were reversed by insulin treatment (3,19). These metabolic effects may have contributed to AP normalization in trained diabetic rats.
Reduction in HR in sedentary diabetic animals has been attributed to changes in the sinoatrial node (2,20), although functional alterations in the cholinergic mechanism cannot be excluded as a causal factor. In the present study we observed an increase in resting HR in trained diabetic rats that was correlated with changes in IHR (r = 0.74), confirming the important role of the sinoatrial node in changes in HR in experimental diabetes. In contrast, previous studies have demonstrated that exercise training decreases resting HR in normotensive rats (21) and humans. The decreased IHR previously observed in our laboratory in trained control rats (11,21) as well as a decreased sympathetic tonus in spontaneously hypertensive rats after training may be the mechanisms involved in exercise bradycardia (10). In the present experiments we did not observe changes in sympathetic tonus between groups, suggesting that the increase in resting HR in trained diabetic rats may be related to the improvement of intrinsic pacemaker regulation. The reduction in vagal tonus indicated a reduction of vagal function in rats with STZ-induced diabetes, probably related to vagal cardiac neuropathy, since a decrease in acetylcholine concentration (22) and a defect in cardiac cholinergic nerves (23) were described. Exercise training did not modify the changes in parasympathetic function of diabetic rats, although a slight increase in vagal tonus was observed in trained rats. Impairment of vagal function evaluated by the reduced bradycardia elicited by increasing AP or electrical vagal stimulation was observed in normal rats after exercise training (21).
The hemodynamic changes induced by diabetes are usually accompanied by myocardial abnormalities in patients (1) and experimental animals (4,13). Although in the present study we did not find differences in left ventricular isovolumetric systolic pressure between groups, cardiac contractility and relaxation, respectively represented by +dP/dt and -dP/dt, were all reduced in hearts isolated from STZ-treated rats, particularly at left ventricular diastolic pressures higher than 10 mmHg. These findings are in agreement with the results reported by DeBlieux et al. (13) showing a slight reduction in cardiac output and no changes in other indices of cardiac work in diabetic rats. However, reduction in myocardial performance has been previously reported (12) and several authors (24,25) have demonstrated that these changes may be related not only to depressed myosin ATPase but also to decreased calcium uptake by the sarcoplasmic reticulum (13). However, the most impressive finding in the present study is that exercise training reverses the changes in contractile properties of the heart induced by STZ-diabetes in rats. Paulson et al. (26) reported that exercise training improved cardiac function in diabetic animals by decreasing the severity of the diabetic state. In the present study the increase in body weight in trained rats seems to indicate metabolic changes. Moreover, it is well known that training is able to increase sensitivity to insulin (9,11). Since STZ-treated rats did not receive insulin treatment in the present study, the improvement in metabolic state was probably due to maintenance of increased insulin sensitivity during the post-exercise period (27). Indeed, exercise training increases whole body insulin sensitivity and glucose oxidation by skeletal and cardiac muscle (28). This improvement may be due to the increase in myocardial sarcolemmal GLUT-4 in the diabetic hearts (14). Changes in myocardial metabolism involving a shift from glucose to fat metabolism (28) have been reported in diabetes mellitus. Hence, rats treated with STZ have increased plasma levels of triglycerides and cholesterol (26) that are lowered by exercise training, leading to improved myocardial sarcoplasmic reticulum function.
Finally, while low-intensity exercise training seems to improve cardiovascular function some types of endurance training can exacerbate the diabetes-induced decrease in myocardial Ca2+-activated ATPase and B-adrenergic receptor number (29). The results of the present study provide evidence for the effectiveness of exercise training in reducing some of the cardiovascular complications associated with diabetes mellitus.
Discussion
Acknowledgments
The authors are grateful to Imbramed Ltda. for its technical support in physical training equipment.
Address for correspondence: M.C. Irigoyen, Unidade de Hipertensão, Instituto do Coração, Av. Enéas de Carvalho Aguiar, 44, 05403-000 São Paulo, SP, Brasil. Fax: +55-11-3069-5048. E-mail: hipirigoyen@incor4.incor.usp.br
Presented at the III International Symposium on Vasoactive Peptides, Belo Horizonte, MG, Brasil, October 8-10, 1999. Research supported by CNPq, CAPES and FAPERGS. Publication supported by FAPESP. Received November 26, 1999. Accepted February 21, 2000.
- 1. Kannel WB (1978). Role of diabetes in cardiac disease: conclusions from population studies. In: Zonaraich S (Editor), Diabetes and Heart Thomas, Springfield, IL, 97-112.
- 2. Maeda CY, Fernandes TG, Timm HB & Irigoyen MC (1995). Autonomic dysfunction in short-term experimental diabetes. Hypertension, 26: 1100-1104.
- 3. Schaan BD, Maeda CY, Timm HB, Medeiros S, Moraes R, Ferlin E, Fernandes TG, Ribeiro JP, Schmid H & Irigoyen MC (1997). Time-course of changes in heart rate and blood pressure in rats with streptozotocin-induced diabetes treated with insulin. Brazilian Journal of Medical and Biological Research, 30: 1081-1086.
- 4. Fein FS, Kornstein LB, Strobeck JE, Capasso JM & Sonnenblick EH (1980). Altered myocardial mechanics in diabetic rats. Circulation Research, 47: 922-933.
- 5. Sampson MJ, Wilson S, Karaggianis P, Edmonds ME & Watkins PJ (1990). Progression of diabetic autonomic neuropathy over a decade in insulin dependent diabetics. Quarterly Journal of Medicine, 75: 635-646.
- 6. Monckton G & Pehowich E (1980). Autonomic neuropathy in the streptozotocin diabetic rat. Journal Canadien des Sciences Neurologiques, 7: 135-142.
- 7. Yagihashi S & Sima AAS (1986). Diabetic autonomic neuropathy in the BB rat: Ultrastructural and morphometric changes in parasympathetic nerves. Diabetes, 34: 558-564.
- 8. Maeda CY, Fernandes TG, Lulhier F & Irigoyen MC (1995). Streptozotocin diabetes modifies arterial pressure and baroreflex sensitivity in rats. Brazilian Journal of Medical and Biological Research, 28: 497-501.
- 9. De Angelis KLD, Oliveira AR, Werner A, Bock P, Belló-Klein A & Irigoyen MC (1997). Exercise training in aging: hemodynamic, metabolic, and oxidative stress evaluations. Hypertension, 30: 767-771.
- 10. Gava NS, Véras-Silva AS, Negrăo NE & Krieger EM (1995). Low-intensity exercise training attenuates cardiac beta-adrenergic tone during exercise in spontaneously hypertensive rats. Hypertension, 26: 1129-1133.
- 11. De Angelis KLD, Gadonski G, Fang J, Dall'Ago P, Albuquerque VL, Peixoto LRA, Fernandes TG & Irigoyen MC (1999). Exercise reverses peripheral insulin resistance in trained L-NAME-hypertensive rats. Hypertension, 34: 768-772.
- 12. Paulson DJ, Stephen JK, Peace DG & Tow JP (1988). Improved post-ischemic recovery of cardiac pump function in exercised trained diabetic rats. Journal of Applied Physiology, 65: 187-193.
- 13. De Blieux PM, Barbee RW, McDonough KH & Shepherd RE (1993). Exercise training improves cardiac performance in diabetic rats. Proceedings of the Society for Experimental Biology and Medicine, 203: 209-213.
- 14. Osborn BA, Darr JT, Laddaga RA, Romano FD & Paulson DJ (1997). Exercise training increases sarcolemmal GLUT-4 protein and mRNA content in diabetic heart. Journal of Applied Physiology, 82: 828-834.
- 15. Tancrede G, Rousseau-Migneron S & Nadeau A (1982). Beneficial effects of physical training in rats with a mild streptozotocin-induced diabetes mellitus. Diabetes, 31: 406-409.
- 16. Jackson CV & Carrier GO (1983). Influence of short-term experimental diabetes on blood pressure and heart rate in response to norepinephrine and angiotensin II in the conscious rat. Journal of Cardiovascular Pharmacology, 5: 260-265.
- 17. Cohen AJ, McCarthy DM & Rosseti RR (1986). Renin secretion by the spontaneously diabetic rat. Diabetes, 35: 341-346.
- 18. Hall JL, Sexton WL & Stanley WC (1995). Exercise training attenuates the reduction in myocardial GLUT-4 in diabetic rats. Journal of Applied Physiology, 78: 76-81.
- 19. Garvey WT, Hardin D, Juhaszova M & Dominguez JH (1993). Effects of diabetes on myocardial glucose transport system in rats: implications for diabetic cardiomyopathy. American Journal of Physiology, 264: H837-H844.
- 20. Dall`Ago P, Fernandes TG, Machado UF, Belló AA & Irigoyen MC (1997). Baroreflex and chemoreflex dysfunction in streptozotocin-diabetic rats. Brazilian Journal of Medical and Biological Research, 30: 119-124.
- 21. Negrăo CE, Moreira ED, Santos MCLM, Farah VMA & Krieger EM (1992). Vagal function impairment after exercise training. Journal of Applied Physiology, 72: 1749-1753.
- 22. Kubtscherova J & Vlk J (1970). Influence of alloxan diabetes on acetylcholine synthesis in tissues of the albino rat. Physiologia Bohemoslovaca, 19: 431-434.
- 23. Tomlison DR & Yusof APM (1983). Autonomic neuropathy in the alloxan-diabetic rats. Journal of Autonomic Pharmacology, 3: 257-263.
- 24. Makino N, Dhalla KS, Elimban V & Dhalla NS (1987). Sarcolemmal Ca2+ transport in streptozotocin-induced diabetic cardiomyopathy in rats. American Journal of Physiology, 253: E202- E207.
- 25. Rodrigues B, Ross JR, Farahbakshian S & McNeill JH (1990). Effects of in vivo and in vitro treatment with L-carnitine on isolated hearts from chronically diabetic rats. Canadian Journal of Physiology and Pharmacology, 68: 1085-1092.
- 26. Paulson DJ, Mathews R, Bowman J & Zhao J (1992). Metabolic effects of treadmill exercise training on the diabetic heart. Journal of Applied Physiology, 73: 265-271.
- 27. King DS, Baldus PJ, Sharp RL, Kesl LD, Feltmeyer TL & Riddle MS (1995). Time course for exercise-induced alterations in insulin action and glucose tolerance in middle-aged people. Journal of Applied Physiology, 78: 17-22.
- 28. Tomlinson KG, Gardiner SM, Hebden RA & Bennett T (1992). Functional consequences of streptozotocin-induced diabetes mellitus, with particular reference to the cardiovascular system. Pharmacological Reviews, 44: 103-150.
- 29. Belcastro NA, Maybank P, Rossiter M & Secord D (1985). Effect of endurance swimming on rat cardiac myofibrillar ATPase with experimental diabetes. Canadian Journal of Physiology, 63: 1202-1205.
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
30 May 2000 -
Date of issue
June 2000
History
-
Received
26 Nov 1999 -
Accepted
21 Feb 2000