The Tensions of Scientific Storytelling
By Roald Hoffmann
Science depends on compelling narratives.
Science depends on compelling narratives.

DOI: 10.1511/2014.109.250
Simplicity pleases the mind. Some scientists claim that equations are likely to be correct because they are simple, or that molecules naturally assume more symmetric arrangements of atoms, or that multistep mechanisms for chemical reactions are less common than concerted one-fell-swoop reactions. What happens when the scientist’s hard work reveals that the equation is messy, the molecule looks like an odd clump of pasta, and the mechanism has at least 17 steps?

Illustration by Tom Dunne.
In my July–August 2000 column, I argued that in such situations telling a story takes the place of simplicity as a pleasing principle. Because narrative is not reducible to mathematics, it is not given its due in our scientific world. Too bad; storytelling is both ancient and deeply human. It is a shared treasure between science and the arts and humanities.
The study of stories is an established field of literary criticism or theory. In Introduction to Narratology, Monika Fludernik defines a narrative:
…a representation of a possible world in a linguistic and/or visual medium, at whose center there are one or several protagonists of an anthropomorphic nature who are existentially anchored in a temporal and spatial sense… It is the experience of these protagonists that narratives focus on, allowing readers to immerse themselves in a different world and in the lives of the protagonists.
Narratologists tend to exclude scientific texts and lectures from their purview because of the requirement that stories have a human or anthropomorphic protagonist. They also point to that distinguishing characteristic of fiction, seemingly absent from scientific papers, that we may, through the author’s imagination, enter another person’s mind.
Having read thousands of chemical papers and listened to hundreds of colleagues’ lectures, I chafe against being ruled out of bounds. In the papers I read and write, I feel stories unfold before me. I react to them emotionally. I sense narrative devices in these articles and lectures, employed both spontaneously and purposefully. Let me try here to tease out some of the overlooked narrative attributes of science.
Does the standard scientific article tell the narrator scientist’s story, or is it nature’s? A ready answer is not forthcoming, in part because there are many qualitatively distinct practices of storytelling, even within the one science of chemistry. One part of our science operates in a discovery mode; for example, determining the mechanism by which penicillin deceives the bacterial cell-wall building apparatus. Another part features creation, more akin to the arts and engineering. Here new molecules, say, an antibiotic or a biodegradable polymer, are made. We are graced in chemistry by everything in between, a heady mix of creation and discovery.
In the aspects of chemistry close to creation, the narrator (more than one in coauthored papers) is overt, the spinner of theories, the sequencer of steps in a chemical reaction. The story may be told slowly, in incremental detail, or the goal may be laid out and the achievement is in the path followed. In discovery-mode stories, the narrator may be more obscured. Stories of discovery tell nature’s story, with the scientist only as the conduit—even though the answers would not be known were it not for the scientist.
A prime example of a chemical narrative, no less striking today than when it was published 20 years ago, is the synthesis of paclitaxel, an effective and widely used antitumor drug. And a devilishly intricate molecule. In 1967, its activity in an extract from the bark of the slow-growing Pacific yew tree (Taxus brevifolia, hence the common chemical name taxol) was first noted by Monroe E. Wall and Mansukh C. Wani of the Research Triangle Institute. The tree takes hundreds of years to mature, and stripping off its bark kills it. To gain realistic use in therapy, taxol would have to be synthesized, or produced “semisynthetically” from a renewable precursor.
Competition to synthesize taxol in the laboratory was slow-paced in the beginning and sped up in the 1990s. In a photo finish with K. C. Nicolaou, then at the Scripps Research Institute, the group of Robert A. Holton of Florida State University was first. In early 1994, Holton’s group published two linked papers reporting the synthesis. The therapeutic motivation is stated in the first sentence of the first paper, as is the challenge the molecule’s baroque structure presents: “The total synthesis of … taxol … has stood for over 20 years as a major challenge for organic chemists.”
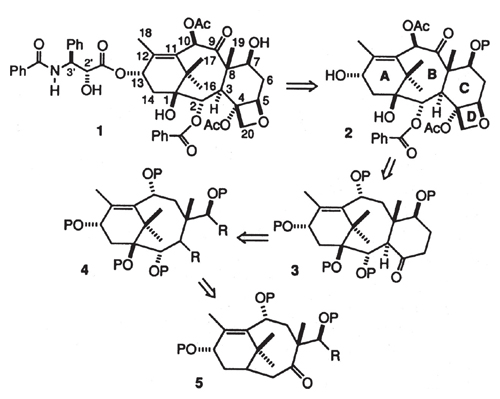
Image from R. A. Holton et al., Journal of the American Chemical Society 116:1597.
Note the establishment of narrative tension—organic chemists had tried before to make taxol and failed to do so. The drawing labeled 1 in the figure at right shows the molecule. Note also the usual organic nomenclature in the molecule’s depiction—a vertex of a polygon is assumed to be a carbon, and the hydrogens attached to it are omitted, but their number is evident if one recognizes that the valence of carbon is normally four.
The authors then voice, modestly but directly, the principal author’s stake in the taxol synthesis:
Until now, our taxane research program has produced a synthesis of the taxane ring system, a total synthesis of taxusin, and a (now commercialized) semisynthesis of taxol.
Presently, the complete synthesis reported in this paper in 1994, a magnificent intellectual achievement, is not used commercially. A “semisynthetic” process, starting with ground-up, farmed ornamental yews, is more efficient. These contain a molecule with most of taxol’s complexity, but lacking the tail at left in 1. The attachment of that tail is covered in a patent referenced in a footnote to the above sentence. That patent, for what seems to be a small piece of the making of a useful molecule, has brought in more than $200 million to Florida State.
Next, Holton and his 17 coworkers get to work:
Thus, our route to taxol proceeds retrosynthetically through C-7 protected baccatin III (2) to the tricyclic ketone 3, which arises from C ring closure of a precursor 4.
The jargon—names of molecules and shorthand for reactions—deals outsiders out, as all jargon does, but nevertheless is assuredly in the toolkit of the readership of this paper.
The word retrosynthetic is key (introduced by the great organic chemist, E. J. Corey of Harvard University): It refers to the structure sequence 1 → 2 → 3 → 4 → 5 shown in the figure above. The thick arrows here mean in plain English “will be derived from,” and the sequence shows conceptual unstitching of the carbon skeleton, progressing from complexity to relative simplicity. The synthetic path the authors contemplate is thus the reverse of this sequence, 5 → 4 → 3 → 2 → 1, where the thin arrows mean “turns into”; it takes many physical steps to achieve each transformation. The beginning, molecule 5, is not as ubiquitous as earth, air, fire, or water, but it is easily available from an abundant natural product, camphor. The actual synthesis, with experimental detail, begins later in the paper. The process takes 37 steps.
Where’s the story here? Well, it’s less of a whodunit and more of a “how-the-heck-did-they-do-it”—in a way, an ultimate demonstration that it is the path that matters. This approach is much like a classic quest narrative. The path is set out in the retrosynthetic chain in the figure on page 252. Chemical “Laistrygonians and Cyclops, angry Poseidon” were along the way; they were overcome or evaded. Will the 18 authors of this paper achieve their goal; will Odysseus reach his Ithaka? They did. And the makers probably would have set out the story—yes, the story—quite differently had this road map failed them.
Note the dual protagonist of this tale: the molecule to be sure, and the chemists who made it. Is not the retrosynthetic scheme, the plan set out to make the molecule, a glimpse into the inner life of the molecule? Or is it the inner thinking of the narrator chemists? Remarkably, the molecule has still another life, another story to tell, one that is not revealed in this paper. It is the way taxol is made, naturally, in the Pacific yew. You can be sure that it isn’t the way that Holton’s group made it. There are six more stories to be told, in the ingenious other syntheses of the molecule.
This paper tells how a much-desired molecule was made for the first time in the laboratory. All the elements of a heroic epic are there—a quest, and in the parts of the paper not shown, battles with the elements, obstacles galore that must be overcome, and in the end, deserved success—with perhaps the exception that the journal article lacks an explicit rendering of the synthetic chemists’ thoughts. But there is enough detail in the story that readers can imagine what the makers felt. One lovely, complex, and useful molecule—breeding a multitude of stories.
In fiction, there is no end to the ways that the author has of posing the narrator—as an omniscient being privy to the thoughts of all the characters, as the inner voice of one protagonist, as a pseudowriter—these are just selections from a repertoire of authorial Russian nesting dolls. Yet even as we recognize the artifice, the author’s métier is to have the reader suspend disbelief. Readers enter into a writer’s machinations to the extent that they forget the author, so eager are they to access the soul of another.
The tension in scientific articles is of another ilk. The protagonists are the investigators of nature. And the investigator takes on two roles. The first is the scientist trying to understand; in his or her mind is a congeries of what teachers taught, what is known. He or she concocts fecund stories of what might be and calls them hypotheses. I refer to that face of a scientist as the “scrabbler,” because attempting to understand anything is a struggle at first. The second face of the scientist is the “writer.” The writer sanitizes, gives the best yield of a reaction, the most plausible story, as mathematically or logically dressed up as possible. Both are narrators—the desire-driven and mistake-prone scrabbler, the oh-so-logical Occam’s Razor–wielding writer. The late Nobel Prize laureate Peter Medawar described the process beautifully in his 1963 lecture “Is the scientific paper a fraud?”
The subject of the scrabbler’s and the writer’s story is reality, represented by the world of science in its ephemeral guise. Represented reality has some observables to throw in the path of the scrabbler who becomes the writer (in a multiauthor paper, each person sometimes takes on the scrabbler role, and other times the writer one). The significance of the facts has to be interpreted. It took us a long time to get past our exquisite yet easily seduced senses, and we need the skeptical rancor of debate to calibrate the reliability of those sensory extensions, our instruments. Carefully done measurements of observables are an essential ingredient of science, against which theories must be measured. They constitute facts, some will say. Well, facts are mute. One needs to situate the facts, or interpret them. To weave them into nothing else but a narrative.
The tension of the scientific narrative resides in the divided personality (or personalities) of the authors, scrabbler and writer, and the representation of reality that their work shapes. Reality turns a different crystal face to all its viewers. With the writer telling the neat story that the stumbling yet imaginative scrabbler found, the investigators together build reality, or a face of reality. That face is in turn seen in a different light by others who compete with, or who follow, the one person who is both scrabbler and writer.
Often the reader is unaware of what lurks beneath the surface of a journal article. There is a prehistory to what is reported. In the course of the work, the authors make decisions that influence how the narrative unfolds. Just as one is interested in how Thomas Mann wrote Death in Venice and, as one reads the novella, what the feelings of the boy Tadzio really are, so one wants to know the story behind the story of a scientific narrative. I only truly know this about my own work, so with the permission of my coauthors, I will tell you the story behind one of my recent papers in the Journal of the American Chemical Society. This may not be the best choice, because I’m a writer too. Like a mild disease, poetry and theater have infected my science writing. So have the strategies of storytelling.
Image from A. Y. Rogachev, X.-D. Wen, and R. Hoffmann, Journal of the American Chemical Society 134:8062.
The article is “Jailbreaking Benzene Dimers,” published in 2012 with two colleagues, Andrey Rogachev and Xiao-Dong Wen. At the time they were postdoctoral researchers in my group; each has now embarked on an independent career. Our paper found its home in an excellent journal, after, incidentally, rejection from another excellent journal. That rejection too can be construed as part of the journey. It made us improve the underlying proofs for our suggested structures, and so made the story stronger.
The story begins with a reference to our previous theoretical work on the behavior of benzene under pressure, which led to the questions we had about benzene dimerization: “In the course of thinking about benzene under gigapascal pressure, we decided we might learn something from the dimers of benzene, as signposts to the pressure-induced polymerization of the compound.”
What we didn’t relate is that there is another, preceding record of chemists experimentally compressing benzene and getting amorphous, hard-to-characterize polymers rather than the nice, orderly structures our group had predicted. Those studies suggested to us that perhaps we should study the first step in any polymerization, and that is the reaction of just two benzene molecules with each other, the dimers. We didn’t rehash the prehistory because it would have taxed the patience of the readers, presumably chemists already familiar with it. As it was, we had a good enough story to tell. The article has hardly the quality of Mann’s novella, but he also chose what to omit, for instance not to tell the reader the previous life of the young boy Tadzio.
After that hint of previous work, we jump right into the current study:
To induce the benzenes to dimerize, we brought two benzene molecules to an uncomfortably close contact, and then let loose the geometry optimization of a quantum chemical program… The molecules reacted to this torture by moving apart, or by forming dimers.
Why did we do that? Because that’s what high pressure does—it forces molecules closer to each other than they “normally” would like to be, much as people do in a subway car at rush hour.
We did not tell our readers that our “explosion” method of finding structures (meaning we put the molecules too close to each other in the simulation, then let them “blow apart,” expand, and in the process explore new bonding arrangements of their component atoms) was already in the literature to study possible arrangements of a given number of atoms. We simply didn’t know that our method was not new; we were led to this procedure for sampling all kinds of bonding by our noses (or rather, Dr. Wen’s nose).
Wen showed me the set of dimers he got from his calculations. Because of my organic chemistry background, I saw that some of his dimers were known, but two were new to me (5 and 6 in the figure above). I said the equivalent of “Play it again, Sam,” and off we went, first continuing the “explosive” way of looking for new benzene dimer structures, and eventually substituting a more systematic exploration as we spotted the essential molecular characteristics of the new molecules.
Where is the dramatic tension here? This work is theory; we predicted a total of four new benzene dimers, and we postdicted seven that are known (and one that people had suggested previously but hasn’t yet been made). The postdiction is actually a good check on our method. We tried very hard to estimate the stability of the predicted new guys. The drama (although I might be biased) is that no one had thought of these four molecules before. Once written down, they seem eminently makeable. But are the calculations behind our predictions good enough? Evidently the reviewers who first rejected our paper—good quantum chemists all, even if I found them illogical for some minutes—thought “no.” One real tension remains unresolved, the classic tension between theory and experiment: Will these molecules actually be made?
Where is the narrative? Before this paper, chemists had synthesized amorphous benzene polymers and seven known dimers. We (both the scrabbler and the writer sides of my coauthors and me) have woven the new structures into a narrative of how a simple dimer is not simple, but rather has 12 realizations (same number of atoms, connected up in a different way; chemists call these isomers of each other). If you saw the scattered pages and computer screen of the theorizing scrabbler’s calculations at the outset of this work, you would never find the story in it. Just numbers. The story behind the story took shape in conversations between my coauthors and me. As writers, we polished up the tale.
The article constructs an inner life for the molecules. We had to worry whether the isomers that we proposed were stable. The molecules’ persistence in the lab depends on the barriers to their falling apart or reacting with other molecules, which are created by bonds, energy levels, and so on. Those barriers are a kind of prison cell; we want the molecules imprisoned, so to speak, because we need time to study them. Ergo the title of the paper—my collaborators (perhaps cursing under their breath at the labor involved) had to look for all the ways in the world by which these dimers could break out of their bond-imposed jail to the greener pastures of lower free energy, a state that all molecules “prefer” to reach.
Science has stories in it. Scientists shape those stories, and the protagonists of these stories need not be human. These narrative qualities are not only important to composing research papers, but also to effective teaching. An innovative, recent chemical text, Mark Green’s Organic Chemistry Principles in Context: A Story Telling Historical Approach, makes consistent use of storytelling by focusing on particular chemical problems and the lives of the chemists who solved these problems.
By analyzing exactly how scientists approach scientific literature, I hope to reveal the humanity of the scientific method. I also aim to demonstrate the connections between the scientific process and other forms of creation, such as art, literature, and storytelling in general, be it Mann’s novella or African Mandé tales. The narrators in chemical articles indeed are human, as much as they may try to efface themselves by writing in the third person. In the literature of chemistry—yes, it is a literature—molecules take on a life of their own, as do the ways of making and identifying them. No anthropomorphization is needed. There is a life-giving tension between the several roles of the scientist as author, revealing and creating onion layers of reality’s representation in his or her science.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.