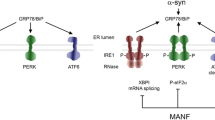
Abstract
Parkinson’s disease (PD) is caused by the degeneration of dopaminergic neurons of substantia nigra projecting to striatum. The cause of idiopathic PD is obscure, and most cases are sporadic. It is widely accepted that there is a genetic component of the disease, and the earlier the age of onset, the greater the likelihood that genetic factors play a dominant role. Oxidative stress of the substantia nigra seems to contain the driving force for neurodegeneration, leading to a destructive “toxic cycle.” The most prevalent therapy is levodopa administration, but it is not efficacious after several years of treatment. Several alternative therapies are currently being explored, such as neuroprotective approaches. Compounds with potentially neuroprotective efficacy such as selegiline, dopamine agonists, riluzole, creatine, and coenzyme Q10 are currently being tested. Trophic factors represent another class of neuroprotective compounds, but their intracerebral administration is difficult to achieve. In this respect, a potentially useful therapeutic approach is grafting cell vectors that release trophic molecules that stimulate regeneration in the damaged nigrostriatal system. Promising results have been obtained with fibroblasts engineered to secrete glial cell line-derived neurotrophic factor (GDNF) or brain-derived neurotrophic factor (BDNF) or viral vectors expressing GDNF. We have tested the suitability of intrastriatal grafts of chromaffin cells obtained from the Zuckerkandl’s organ, which exert beneficial effects in parkinsonian rats, and release trophic factors such as GDNF and transforming growth factor-β1 (TGF-β1).
Similar content being viewed by others
References
Piccini P., Burn D. J., Ceravolo R., Maraganore D., and Brooks D. J. (1999) The role of inheritance in sporadic Parkinson’s disease: evidence from a longitudinal study of dopaminergic functions in twns. Ann. Neurol. 45, 577–582.
Martin W. E., Young W. I., and Anderson V. E. (1973) Parkinson’s disease: a genetic study. Brain 96, 495–506.
Lotharius J., Barg S., Wiekop P., Lundberg C., Raymon H. K., and Brundin P. (2002) Effect of mutant α-synuclein on dopamine homeostasis in a new human mesencephalic cell line. J. Biol. Chem. 277, 38,884–38,894.
Ishikawa A. and Tsuji S. (1996) Clinical analysis of 17 patients in 12 Japanese families with autosomal-recessive type juvenile parkinsonism. Neurology 47, 160–166.
Shimura H., Hattori N., Kubo S., et al. (2000) Familial Parkinson’s disease gene product, parkin, is a ubiquitin-protein ligase. Nat. Genes 25, 302–305.
Mouradian M. M. (2002) Recent advances in the genetics and pathogenesis of Parkinson’s disease. Neurology 58, 179–185.
Seidler A., Hellenbrand W., Robra B. P., et al. (1996) Possible environmental, occupational, and other etiological factors for Parkinson’s disease: a case-control study in Germany. Neurology 46, 1275–1284.
Semchuk K. M., Love E. J., and Lee R. G. (1991) Parkinson’s disease and exposure to rural environmental factors: a population based case-control study. Can. J. Neurol. Sci. 18, 279–286.
Tipton K. F. and Singer T. P. (1993) Advances in our understanding of the mechanisms of the neurotoxicity of MPTP and related compounds. J. Neurochem. 61, 1191–1206.
Langston J. W., Ballard P., Tetrud J. W., and Irwin I. (1983) Chronic parkinsonism in humans due to a product of a meperidine-analog synthesis. Science 219, 979–980.
Shapira A. H. V., Cooper J. M., and Dexter D. (1989) Mithocondrial complex I deficiency in Parkinson’s disease. Lancet 1, 1269.
Beckman J. S., Beckman T. W., Chen J., Marshall P. A., and Freeman B. A. (1990) Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 87, 1620–1624.
Sofic E., Riederer P., Heinsen H., Beckman H., Reynolds G. P., Hebenstreit G., and Youdim M. B. (1988) Increased iron (III) and total iron content in postmortem substantia nigra of parkinsonian brain. J. Neural Trans. 74, 199–205.
Morris C. M. and Edwardson J. A. (1994) Iron histochemistry of the substantia nigra in Parkinson’s disease. Neurodegeneration 3, 277–282.
Pearce R. K., Owen A., Daniel S., Jenner P., and Marsden C. D. (1997) Alterations in the distribution of glutathione in the substantia nigra in Parkinson’s disease. J. Neural Transm. 104, 661–677.
Hunot S., Boissiere F., Faucheux B., Brugg B., Mouatt-Prigent A., Agid Y., and Hirsch E. C. (1996) Nitric oxide synthase and neuronal vulnerability in Parkinson’s disease. Neuroscience 72, 355–363.
Floor E. and Wetzel M. G. (1998) Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J. Neurochem. 70, 2682–2675.
Dexter D. T., Carter C. J., Wells F. R., Javoy-Agid F., Agid Y., Lees A., Jenner P., and Marsden C. D. (1989) Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J. Neurochem. 52, 381–389.
Alam Z. I., Zenner A., Daniel S. A., et al. (1997) Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J. Neurochem. 69, 1196–1203.
Bucciantini M., Giannoni E., Chiti F., et al. (2002) Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 416, 507–511.
Hurtig H. I. Trojanowski J. Q., Galvin J., et al. (2000) α-synuclein cortical Lewy bodies correlate with dementia in Parkinson’s disease. Neurology 54, 1916–1921.
Shermann M. Y. and Goldberg A. (1996) Involvement of molecular chaperones in intracellular protein breakdown. EXS 77, 57–78.
McNaught K. S. P. and Jenner P. (2002) Proteasomal function is impaired in substantia nigra in Parkinson’s disease. Neurosci. Lett. 326, 15–158.
Hartley A., Cooper J. M., and Shapira A. H. (1993) Iron induced oxidative stress and mithocondrial dysfunction: relevance to Parkinson’s disease. Brain Res. 627, 349–353.
Nagatsu T., Mogi M., Ichinose H., and Togari A. (2000) Changes in cytokines and neurotrophins in Parkinson’s disease. J. Neural Trans. 60, 277–290.
Hunot S. and Hirsch E. C. Neuroinflammatory processes in Parkinson’s disease. Ann. Neurol. 53, S49–S60.
Bolam J. P., Freund T. F., Björklund A., Dunnett S. B., and Smith A. D. (1987) Synaptic input and local output of dopaminergic neurons in grafts that functionally reinnervate the host striatum. Exp. Brain Res. 68, 131–146.
Bohn M. C., Cupit L. C., Marciano F., and Gash D. M. (1987) Adrenal medullary grafts enhance recovery of striatal dopaminergic fibers. Science 237, 913–916.
Goetz C. G., Stebbins G. T., Klawans H. L., Holler W. C., Grossman R. G., Bakay R. A., and Penn R. D. (1991) United Parkinson Foundation neurotransplantation registry on adrenal medullary transplants presurgical, and 1-year and 2-year follow-up. Neurology 41, 1719–1722.
Espejo E. F., Montoro R. J., Armengol J. A., and López-Barneo J. (1998) Cellular and functional recovery of parkinsonian rats after intrastriatal transplantation of carotid body cell aggregates. Neuron 20, 197–206.
Luquin M. R., Montoro R. J., Guillén J., Saldise L., Insausti R., Del Río J., and López-Barneo J. (1999) Recovery of chronic parkinsonian monkeys by autotransplants of carotid body cell aggregates into putamen. Neuron 22, 743–750.
Lindvall O. (1997) Neural transplantation: a hope for patients with Parkinson’s disease? NeuroReport 8, iii-x.
Olanow C. W., Freeman T. B., and Kordower J. H. (1997) Neural transplantation as a therapy for Parkinson’s disease. Adv. Neurol. 74, 246–269.
Dunnett S. B. and Björklund A. (1999) Prospects for new restorative and neuroprotective treatments in Parkinson’s disease. Nature 399, A32-A39.
Brundin P. and Hagell P. (2001) The neurobiology of cell transplantation in Parkinson’s disease. Clin. Neurosci. Res. 1, 507–520.
Freed C. R., Greene P. R., Breeze R. E., et al. (2001) Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N. Engl. J. Med. 344, 710–719.
Ma Y., Feigin A., Dhawan V., et al. (2002) Diskynesia after fetal cell transplantation for parkinsonism: a PET study. Ann. Neurol. 52, 628–634.
Yurek D. M. and Sladek J. R. (1990) Dopamine cell replacement: Parkinson’s disease. Annu. Rev. Neurosci. 13, 415–440.
Jenner P. and Olanow C. W. (1998) Understanding cell death in Parkinson’s disease. Ann. Neurol. 44, 72–84.
Cohen G., Pasik P., Cohen B., Leist A., Mytilineou C., and Yahr M. D. (1985) Pargyline and deprenyl prevent the neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in monekys. Eur. J. Pharmacol. 106, 209–210.
Elizan T. S., Yahr M. D., Moros D. A., Mendoza M. R., Pang S., and Bodian C. A. (1989) Selegiline use to prevent progression of Parkinson’s disease. Experience in 22 de novo patients. Arch. Neurol. 46, 1275–1279.
Brannan T. and Yahr M. D. (1995) Comparatives tudy of selegiline plus l-dopa-carbidopa versus l-dopa-carnidopa alone in the treatment of Parkinson’s disease. Ann. Neurol. 37, 95–98.
The Parkinson’s Study Group. (1996) The impact of extended deprenyl and tocopherol treatment in Parkinson’s disease. Ann. Neurol. 39, 29–36.
Shoulson I., Oakes D., Fahn S., et al. (Parkinson Study Group) (2002) The impact of sustained deprenyl (selegiline) in levodopa-treated Parkinson’s disease: a randomized placebo-controlled extension. Ann. Neurol. 51, 604–612.
Ogawa N., Tanaka K., Asanuma M., et al. (1994) Bromocriptine protects mice against 6-hydroxy-dopamine and scavenges hydroxyl free radical in vitro. Brain Res. 657, 207–213.
Muralikrishnan D. and Mohanakumar K. P. (1998) Neuroprotection by bromocriptine against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in mice. FASEB J. 12, 905–912.
Olanow C. W. (1992) A rationale for dopamine agonists as primary therapy for Parkinson’s disease. Can. J. Neurosci. 19, 108–112.
Jenner P., Iravani M. M., Haldon C. O., et al. (2002) Pramipexole protects against MPTP-induced nigral dopaminergic cell loss in primates. Neurology 58, 494.
Whone A. L., Remy P., Davis M. R., et al. (2002) The REAL-PET study: slower progression in early Parkinson’s disease treated with ropinirole compared with l-dopa. Neurology 58, 82–83.
Araki T., Kumagai T., Tanaka K. Matsubara M., Kato H., Itoyama Y., and Imai Y. (2001) Neuroprotective effect of riluzole in MPTP-treated mice. Brain Res. 918, 176–181.
Obinu M. C., Reibaud M., Blanchard V., Moussaouis S., and Imperato A. (2002) Neuroprotective effect of riluzole in a primate model of Parkinson’s disease: behavioral and histological evidence. Mov. Disord. 17, 13–19.
Matthews R. T., Ferrante R. J., Klivenyi P., et al. (1999) Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp. Neurol. 157, 142–149.
Beal M. F., Matthews R. T., Tielemen A., and Shults C. W. (1998) Coenzyme Q10 attenuates the of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced loss of striatal dopamine and dopaminergic axons in aged mice. Brain Res. 783, 109–114.
Shults C. W., Oakes D., Kieburtz K., et al. (Parkinson Study Group) (2002) Effect of coenzyme Q10 in early Parkinson’s disease: Evidence of slowing of the functional decline. Arch. Neurol. 59, 1541–1550.
Collier T. and Sorwell C. E. (1999) Therapeutic potential of nerve growth factors in Parkinson’s disease. Drugs Aging 14, 261–287.
Hoffer B. J., Hoffman A., Bowenkamp K., et al. (1994) Glial cell line-derived neurotrophic factor reverses toxin-induced injury to midbrain dopaminergic neurons in vivo. Neurosci. Lett. 182, 107–111.
Hebert M. A., Hoffer B. J., and Zhang Z. (1999) Functional effects of GDNF in normal and parkinsonian rats and monkeys, In: CNS Regeneration: Basic Science and Clinical Advances (Tuszynski M., and Kordower J. H., eds.), Academic Press, New York, pp. 419–436.
Kordower J. H., Palfi S., Chen E., et al. (1999) Clinicopathological findings following intraventricular glial-derived neurotrophic factor treatment in a patient with Parkinson’s disease. Ann. Neurol. 46, 419–424.
Kordower J. H. (2003) In vivo gene delivery of glial cell line-derived neurotrophic factor for Parkinson’s disease. Ann. Neurol. 53, S120-S134.
Stocchi F. and Olanow C. W. (2003) Neuroprotection in Parkinson’s disease: clinical trials. Ann. Neurol. 53, S87-S99.
Lin L. F., Doherty D. H., Lile J. D., Bektesh S., and Collins F. (1993) GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260, 1130–1132.
Tomac A., Lindqvist E., Lin L. F., Ogren S. O., Young D., Hoffer B. J., and Olson L. (1995) Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature 373, 335–339.
Beck K. D., Valverde J., Alexi T., et al. (1995) Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature 373, 339–341.
Gash D. M., Zhang Z. M., and Gerhardt G. (1998) Neuroprotective and neurorestorative properties of GDNF. Ann. Neurol. 44, G121-S125.
Tseng J. L., Baetge E. E., Zurn A. D., and Aebisher P. (1997) GDNF reduces drug-induced rotational behavior after medial forebrain bundle transection by a mechanisms not involving striatal dopamine. J. Neurosci. 17, 325–333.
Levivier M., Przedborski S., Bencsics C., and Kang U. (1995) Intrastriatal transplantation of fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevents degeneration of dopaminergic neurons in a rat model of Parkinson’s disease. J. Neurosci. 15, 7810–7820.
Frim D. M., Uhler T. A., Galpern W. R., Beal M. F., Breakfield X. O., and Isacson O. (1994) Implanted fibroblasts genetically engineered to produce brain derived neurotrophic factor prevent 1-methyl-4-phenylpyridinium toxicity to dopaminergic neurons in the rat. Proc. Natl. Acad. Sci. USA 91, 5104–5108.
Mandel R. J., Spratt S. K., Snyder R. O., and Leff S. E. (1997) Midbrain injection of recombinant adeno-associated virus encoding rat glial cell line-derived neurotrophic factor protects nigral neurons in a progressive 6-hydroxydopamine-induced degeneration model of Parkinson’s disease in rats. Proc. Natl. Acad. Sci. USA 94, 14,083–14,088.
Kirik D., Rosenblad C., Björklund A., and Mandel R. J. (2000) Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson’s model: intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J Neurosci. 20, 4686–4700.
Unsicker K., Suter-Crazzolara C., and Krieglstein K. (1996) Growth factor function in the development and maintenance of midbrain dopaminergic neurons: concepts, facts and prospects for TGF-β. Ciba Found. Symp. 196, 70–80.
Krieglstein K., Henheik P., Farkas L., Jaszai J., Galter D., Krohn K., and Unsicker K. (1998) Glia cell line-derived neurotrophic factor requires transforming growth factor-beta for exerting its full neurotrophic potential on peripheral an CNS neurons. J. Neurosci. 18, 9822–9834.
Schober A., Hertel R., Arumae U., et al. (1999) Glial cell line-derived neurotrophic factor rescues target-deprived sympathetic spinal cord neurons but requires transforming growth factor-beta as cofactor in vivo. J. Neurosci. 19, 2008–2015.
Freed W. J., Morihisa J. M., Spoor E., Hoffer B. J., Olson L., Seiger A., and Wyatt R. J. (1981) Transplanted adrenal chromaffin cells in rat brain reduce lesion-induced rotational behavior. Nature 292, 351–352.
Unsicker K. and Krieglstein K. (1995) Bovine chromaffin cells release a transforming growth factor-beta-like molecule contained within chromaffin granules. J. Neurochem. 65, 1423–1426.
Unsicker K. and Krieglstein K. (1996) Growth factors in chromaffin cells. Prog. Neurobiol. 48, 307–324.
O’Connor D. T. (1999) Chromaffin cell mechanisms: understanding catecholamine storage and release. Trends Pharmacol. Sci. 20, 431–432.
Toledo-Aral J. J., Mendez-Ferrer S., Pardal R., Echevarria M., Lopez-Barneo J. (2003) Trophic restoration of the nigrostriatal dopaminergic pathway in long-term carotid body-grafted parkinsonian rats. J. Neurosci. 23, 141–148.
Ahonen M., Soinila S., and Joh T. H. (1987) Preand postnatal development of rat retroperitoneal paraganglia. J Auton. Nerv. Syst. 18, 11–120.
Testut L. and Latarjet A. (1978) Tratado de anatomía humana. Salvat, Barcelona.
Espejo E. F., González-Albo M. C., Moraes J. P., El Banoua F., Flores J. A., and Caraballo I. (2001) Functional regeneration in a rat Parkinson’s model after intrastriatal grafts of GDNF and TGF-β1-expressing extra-adrenal chromaffin cells of the Zuckerkandl’s organ. J. Neuroscience 21, 9888–9895.
Bohn M. C., Goldstein M., and Black I. B. (1982) Expression of phenylethanolamine N-methyl-transferase in rat sympathetic ganglia and extra-adrenal chromaffin tissue. Develop. Biol. 89, 299–308.
Fornaguera J., Carey R. J., Huston J. P., and Schwarting R. K. W. (1994) Behavioral asymmetries and recovery in rats with different degrees of unilateral striatal dopamine depletion. Brain Res. 664, 178–188.
Schwarting R. K. W. and Huston J. P. (1996) The unilateral 6-hydroxydopamine lesion model in behavioral brain research: analysis of functional deficits, recovery and treatments. Prog. Neurobiol. 50, 275–331.
Björklund A., Dunnett S. B., Stenevi U., Lewis M. E., and Iversen S. D. (1980) Reinnervation of the denervated striatum by substantia nigra transplants: functional consequences as revealed by pharmacological and sensorimotor testing. Brain Res. 199, 307–333.
Brundin P., Strecker R. E., Londos E., and Björklund A. (1987) Dopamine neurons grafted unilaterally to the nucleus accumbens affect drug-induced circling and locomotion. Exp. Brain Res. 69, 183–194.
Brundin P., Karlsson J., Emgard M., et al. (2000) Improving the survival of grafted dopaminergic neurons: a review over current approaches. Cell Transpl. 9, 179–195.
Lyon R. A., Titeler M., Bigornia L., and Schneider A. S. (1987) D2 dopamine receptors on bovine chromaffin cell membranes: identification and characterization by [3H]N-methyl-spiperone binding. J. Neurochem. 48, 631–635.
Missale C., Castelleti L., Memo M., Carruba M. O., and Spano P. F. (1988) Identification of postsynaptic D1 and D2 dopamine receptors in cardiovascular system. J. Cardiovasc. Pharmacol. 11, 643–650.
Pupilli C., Lanzillotti R., Fiorelli G., et al. (1994) Dopamine D2 receptors gene expression and binding sites in adrenal medulla and pheocromocytoma. J. Clin. Endocrinol. Metab. 79, 56–61.
Unsicker K. (1993) The trophic cocktail made by adrenal chromaffin cells. Exp. Neurol. 123, 167–173.
Jennings C. (2000) Is neural cell transplantation ready for the clinic? Nature Medicine 6, 634.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fernandez-Espejo, E. Pathogenesis of parkinson’s disease. Mol Neurobiol 29, 15–30 (2004). https://doi.org/10.1385/MN:29:1:15
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/MN:29:1:15