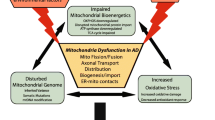
Abstract
Oxidative stress is a striking feature of susceptible neurons in the Alzheimer’s disease brain. Importantly, because oxidative stress is an early event in Alzheimer’s disease, proximal to the development of hallmark pathologies, it likely plays an important role in the pathogenesis of the disease. Investigations into the cause of such oxidative stress show that interactions between abnormal mitochondria and disturbed metal metabolism are, at least in part, responsible for cytoplasmic oxidative damage observed in these susceptible neurons, which could ultimately lead to their demise. Oxidative stress not only temporally precedes the pathological lesions of the disease but could also contribute to their formation, which, in turn, could provide some protective mechanism to reduce oxidative stress and ensure that neurons do not rapidly succumb to oxidative insults. In this review, we present the evidence for oxidative stress in Alzheimer’s disease and its likely sources and consequence in relation to other pathological changes.
Similar content being viewed by others
References
Halliwell B. (1992) Reactive oxygen species and the central nervous system. J. Neurochem. 59, 1609–1623.
Joseph J., Shukitt-Hale B., Denisova N.A., et al. (2001) Copernicus revisited: amyloid beta in Alzheimer’s disease. Neurobiol. Aging 22, 131–146.
Hirai K., Aliev G., Nunomura A., et al. (2001) Mitochondrial abnormalities in Alzheimer’s disease. J. Neurosci. 21, 3017–3023.
Zhu X., Raina A.K., Perry G., and Smith M.A. (2004) Alzheimer’s disease: the two-hit hypothesis. Lancet Neurol. 3, 219–226.
Smith M.A., Rottkamp C.A., Nunomura A., et al. (2000) Oxidative stress in Alzheimer’s disease. Biochim. Biophys. Acta 1502, 139–144.
Good P.F., Werner P., Hsu A., et al. (1996) Evidence of neuronal oxidative damage in Alzheimer’s disease. Am. J. Pathol. 149, 21–28.
Smith M.A., Richey Harris P.L., Sayre L.M., et al. (1997) Widespread peroxynitrite-mediated damage in Alzheimer’s disease. J. Neurosci. 17, 2653–2657.
Smith M.A., Perry G., Richey P.L., et al. (1996) Oxidative damage in Alzheimer’s. Nature 382, 120–121.
Castegna A., Thongboonkerd V., Klein J.B., et al. (2003) Proteomic identification of nitrated proteins in Alzheimer’s disease brain. J. Neurochem. 85, 1394–1401.
Williamson K.S., Gabbita S.P., Mou S., et al. (2002) The nitration product 5-nitro-gamma-tocopherol is increased in the Alzheimer brain. Nitric Oxide 6, 221–227.
Sayre L.M., Smith M.A., and Perry G. (2001) Chemistry and biochemistry of oxidative stress in neurodegenerative disease. Curr. Med. Chem. 8, 721–738.
Smith M.A., Sayre L.M., Anderson V.E., et al. (1998) Cytochemical demonstration of oxidative damage in Alzheimer disease by immunochemical enhancement of the carbonyl reaction with 2,4-dinitrophenylhydrazine. J. Histochem. Cytochem. 46, 731–735.
Montine T.J., Amarnath V., Martin M.E., et al. (1996) E-4-Hydroxy-2-nonenal is cytotoxic and cross-links cytoskeletal proteins in P19 neuroglial cultures. Am. J. Pathol. 148, 89–93.
Sayre L.M., Zelasko D.A., Harris P.L., et al. (1997) 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J. Neurochem. 68, 2092–2097.
Nunomura A., Perry G., Pappolla M.A., et al. (1999) RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J. Neurosci. 19, 1959–1964.
Rottkamp C.A., Nunomura A., Raina A.K., et al. (2000) Oxidative stress, antioxidants, and Alzheimer disease. Alzheimer Dis. Assoc. Disord. 14(Suppl. 1), S62-S66.
Rinaldi P., Polidori M.C., Metastasio A., et al. (2003) Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol. Aging 24, 915–919.
Riviere S., Birlouez-Aragon I., Nourhashemi F., and Vellas B. (1998) Low plasma vitamin C in Alzheimer patients despite an adequate diet. Int. J. Geriatr. Psychiatry 13, 749–754.
Bourdel-Marchasson I., Delmas-Beauvieux M.C., Peuchant E., et al. (2001) Antioxidant defences and oxidative stress markers in erythrocytes and plasma from normally nourished elderly Alzheimer patients. Age Ageing 30, 235–241.
Lovell M.A., Xie C., and Markesbery W.R. (1998) Decreased glutathione transferase activity in brain and ventricular fluid in Alzheimer’s disease. Neurology 51, 1562–1566.
Riviere S., Birlouez-Aragon I., and Vellas B. (1998) Plasma protein glycation in Alzheimer’s disease. Glycoconj. J. 15, 1039–1042.
Pratico D. (2002) Alzheimer’s disease and oxygen radicals: new insights. Biochem. Pharmacol. 63, 563–567.
Markesbery W.R. and Carney J.M. (1999) Oxidative alterations in Alzheimer’s disease. Brain Pathol. 9, 133–146.
Markesbery W.R. and Lovell M.A. (1998) Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer’s disease. Neurobiol. Aging 19, 33–36.
Pratico D., Trojanowski J.Q., Rokach J., et al. (1998) Increased F2-isoprostanes in Alzheimer’s disease: evidence for enhanced lipid peroxidation in vivo. FASEB J. 12, 1777–1783.
Montine T.J., Beal M.F., Cudkowicz M.E., et al. (1999) Increased CSF F2-isoprostane concentration in probable AD. Neurology 52, 562–565.
Montine T.J., Sidell K.R., Crews B.C., et al. (1999) Elevated CSF prostaglandin E2 levels in patients with probable AD. Neurology 53, 1495–1498.
Montine T.J., Markesbery W.R., Zackert W., et al. (1999) The magnitude of brain lipid peroxidation correlates with the extent of degeneration but not with density of neuritic plaques or neurofibrillary tangles or with APOE genotype in Alzheimer’s disease patients. Am. J. Pathol. 155, 863–868.
Pratico D., Clark C.M., Lee V.M., et al. (2000) Increased 8,12-iso-iPF2alpha-VI in Alzheimer’s disease: correlation of a noninvasive index of lipid peroxidation with disease severity. Ann. Neurol. 48, 809–812.
Tuppo E.E., Forman L.J., Spur B.W., et al. (2001) Sign of lipid peroxidation as measured in the urine of patients with probable Alzheimer’s disease. Brain Res. Bull. 54, 565–568.
Pratico D., Clark C.M., Liun F., et al. (2002) Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch. Neurol. 59, 972–976.
Lovell M.A., Gabbita S.P., and Markesbery W.R. (1999) Increased DNA oxidation and decreased levels of repair products in Alzheimer’s disease ventricular CSF. J. Neurochem. 72, 771–776.
Tohgi H., Abe T., Yamazaki K., et al. (1998) The cerebrospinal fluid oxidized NO metabolites, nitrite and nitrate, in Alzheimer’s disease and vascular dementia of Binswanger type and multiple small infarct type. J. Neural Transm. 105, 1283–1291.
Tohgi H., Abe T., Yamazaki K., et al. (1999) Alterations of 3-nitrotyrosine concentration in the cerebrospinal fluid during aging and in patients with Alzheimer’s disease. Neurosci. Lett. 269, 52–54.
Perry G., Castellani R.J., Smith M.A., et al. (2003) Oxidative damage in the olfactory system in Alzheimer’s disease. Acta Neuropathol. (Berl.) 106, 552–556.
Mecocci P., Polidori M.C., Ingegni T., et al. (1998) Oxidative damage to DNA in lymphocytes from AD patients. Neurology 51, 1014–1017.
Mecocci P., Polidori M.C., Cherubini A., et al. (2002) Lymphocyte oxidative DNA damage and plasma antioxidants in Alzheimer disease. Arch. Neurol. 59, 794–798.
Gibson G.E., Pulsinelli W., Blass J.P., and Duffy T.E. (1981) Brain dysfunction in mild to moderate hypoxia. Am. J. Med. 70, 1247–1254.
Blass J.P. and Gibson G.E. (1999) Cerebrometabolic aspects of delirium in relationship to dementia. Dement. Geriatr. Cogn. Disord. 10, 335–338.
Small G.W., Mazziotta J.C., Collins M.T., et al. (1995) Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA 273, 942–947.
Reiman E.M., Caselli R.J., Yun L.S., et al. (1996) Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N. Engl. J. Med. 334, 752–758.
Ibanez V., Pietrini P., Alexander G.E., et al. (1998) Regional glucose metabolic abnormalities are not the result of atrophy in Alzheimer’s disease. Neurology 50, 1585–1593.
Gibson G.E., Sheu K.F., and Blass J.P. (1998) Abnormalities of mitochondrial enzymes in Alzheimer disease. J. Neural Transm. 105, 855–870.
Chandrasekaran K., Giordano T., Brady D.R., et al. (1994) Impairment in mitochondrial cytochrome oxidase gene expression in Alzheimer disease. Brain Res. Mol. Brain Res. 24, 336–340.
Cottrell D.A., Blakely E.L., Johnson M.A., et al. (2001) Mitochondrial enzyme-deficient hippocampal neurons and choroidal cells in AD. Neurology 57, 260–264.
Maurer I., Zierz S., and Moller H.J. (2000) A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol. Aging 21, 455–462.
Nagy Z., Esiri M.M., LeGris M., and Matthews P.M. (1999) Mitochondrial enzyme expression in the hippocampus in relation to Alzheimer-type pathology. Acta Neuropathol. (Berl.) 97, 346–354.
Parker W.D., Jr., Mahr N.J., Filley C.M., et al. (1994) Reduced platelet cytochrome c oxidase activity in Alzheimer’s disease. Neurology 44, 1086–1090.
Parker W.D., Jr., Parks J., Filley C.M., and Kleinschmidt-DeMasters B.K. (1994) Electron transport chain defects in Alzheimer’s disease brain. Neurology 44, 1090–1096.
Gibson G.E., Haroutunian V., Zhang H., et al. (2000) Mitochondrial damage in Alzheimer’s disease varies with apolipoprotein E genotype. Ann. Neurol. 48, 297–303.
Lovell M.A., Ehmann W.D., Butler S.M., and Markesbery W.R. (1995) Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer’s disease. Neurology 45, 1594–1601.
Kulkarni-Narla A., Getchell T.V., Schmitt F.A., and Getchell M.L. (1996) Manganese and copper-zinc superoxide dismutases in the human olfactory mucosa: increased immunoreactivity in Alzheimer’s disease. Exp. Neurol. 140, 115–125.
De Leo M.E., Borrello S., Passantino M., et al. (1998) Oxidative stress and overexpression of managanese superoxide dismutase in patients with Alzheimer’s disease. Neurosci. Lett. 250, 173–176.
Ozcankaya R. and Delibas N. (2002) Malondialdehyde, superoxide dismutase, melatonin, iron, copper, and zinc blood concentrations in patients with Alzheimer disease: cross-sectional study. Croat. Med. J. 43, 28–32.
Aksenov M.Y., Tucker H.M., Nair P., et al. (1998) The expression of key oxidative stress-handling genes in different brain regions in Alzheimer’s disease. J. Mol. Neurosci. 11, 151–164.
Serra J.A., Dominguez R.O., de Lustig E.S., et al. (2001) Parkinson’s disease is associated with oxidative stress: comparison of peripheral antioxidant profiles in living Parkinson’s, Alzheimer’s and vascular dementia patients. J. Neural Transm. 108, 1135–1148.
Gulesserian T., Seidl R., Hardmeier R., et al. (2001) Superoxide dismutase SOD1, encoded on chromosome 21, but not SOD2 is overexpressed in brains of patients with Down syndrome. J. Invest. Med. 49, 41–46.
Behl C. (1997) Amyloid beta-protein toxicity and oxidative stress in Alzheimer’s disease. Cell Tissue Res. 290, 471–480.
Markesbery W.R. (1997) Oxidative stress hypothesis in Alzheimer’s disease. Free Radical Biol. Med. 23, 134–147.
Gsell W., Conrad R., Hickethier M., et al. (1995) Decreased catalase activity but unchanged superoxide dismutase activity in brains of patients with dementia of Alzheimer type. J. Neurochem. 64, 1216–1223.
Takahashi M., Dore S., Ferris C.D., et al. (2000) Amyloid precursor proteins inhibit heme oxygenase activity and augment neurotoxicity in Alzheimer’s disease. Neuron 28, 461–473.
Behl C., Davis J.B., Lesley R., and Schubert D. (1994) Hydrogen peroxide mediates amyloid beta protein toxicity. Cell 77, 817–827.
Samudralwar D.L., Diprete C.C., Ni B.F., et al. (1995) Elemental imbalances in the olfactory pathway in Alzheimer’s disease. J. Neurol. Sci. 130, 139–145.
Smith M.A., Harris P.L., Sayre L.M., and Perry G. (1997) Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc. Natl. Acad. Sci. USA 94, 9866–9868.
Deibel M.A., Ehmann W.D., and Markesbery W.R. (1996) Copper, iron, and zinc imbalances in severely degenerated brain regions in Alzheimer’s disease: possible relation to oxidative stress. J. Neurol. Sci. 143, 137–142.
Smith M.A., Wehr K., Harris P.L., et al. (1998) Abnormal localization of iron regulatory protein in Alzheimer’s disease. Brain Res. 788, 232–236.
Connor J.R., Snyder B.S., Arosio P., et al. (1995) A quantitative analysis of isoferritins in select regions of aged, parkinsonian, and Alzheimer’s diseased brains. J. Neurochem. 65, 717–724.
Loeffler D.A., Connor J.R., Juneau P.L., et al. (1995) Transferrin and iron in normal, Alzheimer’s disease, and Parkinson’s disease brain regions. J. Neurochem. 65, 710–724.
Kato J. and Niitsu Y. (2002) Recent advance in molecular iron metabolism: translational disorders of ferritin. Int. J. Hematol. 76, 208–212.
Pinero D.J., Hu J. and Connor J.R. (2000) Alterations in the interaction between iron regulatory proteins and their iron responsive element in normal and Alzheimer’s diseased brains. Cell. Mol. Biol. (Noisy-le-grand). 46, 761–776.
Sayre L.M., Perry G., Harris P.L., et al. (2000) In situ oxidative catalysis by neurofibrillary tangles and senile plaques in Alzheimer’s disease: a central role for bound transition metals. J. Neurochem. 74, 270–279.
Ding Q. and Keller J.N. (2003) Does proteasome inhibition play a role in mediating neuropathology and neuron death in Alzheimer’s disease? J. Alzheimer’s Dis. 5, 241–245.
Raina A.K., Zhu X., Rottkamp C.A., et al. (2000) Cyclin’ toward dementia: cell cycle abnormalities and abortive oncogenesis in Alzheimer disease. J. Neurosci. Res. 61, 128–133.
Zhu X., Raina A.K., and Smith M.A. (1999) Cell cycle events in neurons. Proliferation or death? Am. J. Pathol. 155, 327–329.
Nagy Z., Esiri M.M., Cato A.M., and Smith A.D. (1997) Cell cycle markers in the hippocampus in Alzheimer’s disease. Acta Neuropathol. (Berl.) 94, 6–15.
Vincent I., Jicha G., Rosado M., and Dickson D.W. (1997) Aberrant expression of mitotic cdc2/cyclin B1 kinase in degenerating neurons of Alzheimer’s disease brain. J. Neurosci. 17, 3588–3598.
Yang Y., Geldmacher D.S., and Herrup K. (2001) DNA replication precedes neuronal cell death in Alzheimer’s disease. J. Neurosci. 21, 2661–2668.
Zhu X., McShea A., Harris P.L., et al. (2004) Elevated expression of a regulator of the G2/M phase of the cell cycle, neuronal CIP-1-associated regulator of cyclin B, in Alzheimer’s disease. J. Neurosci. Res. 75, 698–703.
Barni S., Sciola L., Spano A., and Pippia P. (1996) Static cytofluorometry and fluorescence morphology of mitochondria and DNA in proliferating fibroblasts. Biotech. Histochem. 71, 66–70.
Bowser R. and Smith M.A. (2002) Cell cycle proteins in Alzheimer’s disease: plenty of wheels but no cycle. J. Alzheimer’s Dis. 4, 249–254.
Smith M.A., Richey P.L., Taneda S., et al. (1994) Advanced Maillard reaction end products, free radicals, and protein oxidation in Alzheimer’s disease. Ann. NY Acad. Sci. 738, 447–454.
Smith M.A., Rudnicka-Nawrot M., Richey P.L., et al. (1995) Carbonyl-related posttranslational modification of neurofilament protein in the neurofibrillary pathology of Alzheimer’s disease. J. Neurochem. 64, 2660–2666.
Smith M.A., Taneda S., Richey P.L., et al. (1994) Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc. Natl. Acad. Sci. USA 91, 5710–5714.
Vitek M.P., Bhattacharya K., Glendening J.M., et al. (1994) Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc. Natl. Acad. Sci. USA 91, 4766–4770.
Castellani R.J., Harris P.L., Sayre L.M., et al. (2001) Active glycation in neurofibrillary pathology of Alzheimer disease: N(epsilon)-(carboxymethyl) lysine and hexitol-lysine. Free Radical Biol. Med. 31, 175–180.
Nunomura A., Perry G., Aliev G., et al. (2001) Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 60, 759–767.
Nunomura A., Perry G., Pappolla M.A., et al. (2000) Neuronal oxidative stress precedes amyloid-beta deposition in Down syndrome. J. Neuropathol. Exp. Neurol. 59, 1011–1017.
Odetti P., Angelini G., Dapino D., et al. (1998) Early glycoxidation damage in brains from Down’s syndrome. Biochem. Biophys. Res. Commun. 243, 849–851.
Pratico D., Uryu K., Leight S., et al. (2001) Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J. Neurosci. 21, 4183–4187.
Smith M.A., Hirai K., Hsiao K., et al. (1998) Amyloid-beta deposition in Alzheimer transgenic mice is associated with oxidative stress. J. Neurochem. 70, 2212–2215.
Olivieri G., Baysang G., Meier F., et al. (2001) N-acetyl-l-cysteine protects SHSY5Y neuroblastoma cells from oxidative stress and cell cytotoxicity: effects on beta-amyloid secretion and τ phosphorylation. J. Neurochem. 76, 224–233.
Misonou H., Morishima-Kawashima M., and Ihara Y. (2000) Oxidative stress induces intracellular accumulation of amyloid beta-protein (Abeta) in human neuroblastoma cells. Biochemistry 39, 6951–6959.
Frederikse P.H., Garland D., Zigler J.S., Jr., and Piatigorsky J. (1996) Oxidative stress increases production of beta-amyloid precursor protein and beta-amyloid (Abeta) in mammalian lenses, and Abeta has toxic effects on lens epithelial cells. J. Biol. Chem. 271, 10,169–10,174.
Tamagno E., Bardini P., Obbili A., et al. (2002) Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol. Dis. 10, 279–288.
Dyrks T., Dyrks E., Hartmann T., et al. (1992) Amyloidogenicity of beta A4 and beta A4-bearing amyloid protein precursor fragments by metal-catalyzed oxidation. J. Biol. Chem. 267, 18,210–18,217.
Atwood C.S., Moir R.D., Huang X., et al. (1998) Dramatic aggregation of Alzheimer abeta by Cu(II) is induced by conditions representing physiological acidosis. J. Biol. Chem. 273, 12,817–12,826.
Ono K., Hasegawa K., Naiki H., and Yamada M. (2004) Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J. Neurosci. Res. 75, 742–750.
Frautschy S.A., Hu W., Kim P., et al. (2001) Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol. Aging 22, 993–1005.
Pappolla M., Bozner P., Soto C., et al. (1998) Inhibition of Alzheimer beta-fibrillogenesis by melatonin. J. Biol. Chem. 273, 7185–7188.
Hall E.D. and Braughler J.M. (1986) Role of lipid peroxidation in post-traumatic spinal cord degeneration: a review. Central Nerv. Syst. Trauma 3, 281–294.
Kitagawa K., Matsumoto M., Oda T., et al. (1990) Free radical generation during brief period of cerebral ischemia may trigger delayed neuronal death. Neuroscience 35, 551–558.
Jenner P., Dexter D.T., Sian J., et al. (1992) Oxidative stress as a cause of nigral cell death in Parkinson’s disease and incidental Lewy body disease. The Royal Kings and Queens Parkinson’s Disease Research Group. Ann. Neurol. 32(Suppl.), S82-S87.
Geddes J.W., Tekirian T.L., Soultanian N.S., et al. (1997) Comparison of neuropathologic criteria for the diagnosis of Alzheimer’s disease. Neurobiol. Aging 18, S99-S105.
Gentleman S.M., Graham D.I., and Roberts G.W. (1993) Molecular pathology of head trauma: altered beta APP metabolism and the aetiology of Alzheimer’s disease. Prog. Brain. Res. 96, 237–246.
Roberts G.W., Gentleman S.M., Lynch A., et al. (1994) Beta amyloid protein deposition in the brain after severe head injury: implications for the pathogenesis of Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry. 57, 419–425.
Lim G.P., Chu T., Yang F., et al. (2001) The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 21, 8370–8377.
Sung S., Yao Y., Uryu K., et al. (2004) Early vitamin E supplementation in young but not aged mice reduces Abeta levels and amyloid deposition in a transgenic model of Alzheimer’s disease. FASEB J. 18, 323–325.
Matsubara E., Bryant-Thomas T., Pacheco Quinto J., et al. (2003) Melatonin increases survival and inhibits oxidative and amyloid pathology in a transgenic model of Alzheimer’s disease. J. Neurochem. 85, 1101–1108.
Veurink G., Liu D., Taddei K., et al. (2003) Reduction of inclusion body pathology in ApoE-deficient mice fed a combination of antioxidants. Free Radical Biol. Med. 34, 1070–1077.
Johnson G.V. and Bailey C.D. (2002) T, where are we now? J. Alzheimer’s Dis. 4, 375–398.
Gomez-Ramos A., Diaz-Nido J., Smith M.A., et al. (2003) Effect of the lipid peroxidation product acrolein on τ phosphorylation in neural cells. J. Neurosci. Res. 71, 863–870.
Ho P.I., Ortiz D., Rogers E., and Shea T.B. (2002) Multiple aspects of homocysteine neurotoxicity: glutamate excitotoxicity, kinase hyperactivation and DNA damage. J. Neurosci. Res. 70, 694–702.
Olivieri G., Brack C., Muller-Spahn F., et al. (2000) Mercury induces cell cytotoxicity and oxidative stress and increases beta-amyloid secretion and τ phosphorylation in SHSY5Y neuroblastoma cells. J. Neurochem. 74, 231–236.
Mattson M.P., Fu W., Waeg G., and Uchida K. (1997) 4-Hydroxynonenal, a product of lipid peroxidation, inhibits dephosphorylation of the microtubule-associated protein τ. Neuroreport. 8, 2275–2281.
Egana J.T., Zambrano C., Nunez M.T., et al. (2003) Iron-induced oxidative stress modify τ phosphorylation patterns in hippocampal cell cultures. Biometals 16, 215–223.
Ko L., Odawara T., and Yen S.H. (1997) Menadione-induced τ dephosphorylation in cultured human neuroblastoma cells. Brain Res. 760, 118–128.
Davis D.R., Anderton B.H., Brion J.P., et al. (1997) Oxidative stress induces dephosphorylation of τ in rat brain primary neuronal cultures. J. Neurochem. 68, 1590–1597.
Takeda A., Smith M.A., Avila J., et al. (2000) In Alzheimer’s disease, heme oxygenase is coincident with Alz50, an epitope of τ induced by 4-hydroxy-2-nonenal modification. J. Neurochem. 75, 1234–1241.
Perez M., Hernandez F., Gomez-Ramos A., et al. (2002) Formation of aberrant phosphotau fibrillar polymers in neural cultured cells. Eur. J. Biochem. 269, 1484–1489.
Perez M., Cuadros R., Smith M.A., et al. (2000) Phosphorylated, but not native, τ protein assembles following reaction with the lipid peroxidation product, 4-hydroxy-2-nonenal. FEBS Lett. 486, 270–274.
Wilson D.M. and Binder L.I. (1997) Free fatty acids stimulate the polymerization of τ and amyloid beta peptides. In vitro evidence for a common effector of pathogenesis in Alzheimer’s disease. Am. J. Pathol. 150, 2181–2195.
Gamblin T.C., King M.E., Dawson H., et al. (2000) In vitro polymerization of τ protein monitored by laser light scattering: method and application to the study of FTDP-17 mutants. Biochemistry 39, 6136–6144.
Nacharaju P., Lewis J., Easson C., et al. (1999) Accelerated filament formation from τ protein with specific FTDP-17 missense mutations. FEBS Lett. 447, 195–199.
Gamblin T.C., King M.E., Kuret J., et al. (2000) Oxidative regulation of fatty acid-induced τ polymerization. Biochemistry 39, 14,203–14,210.
Smith M.A. (1998) Alzheimer disease. Int. Rev. Neurobiol. 42, 1–54.
Smith M.A., Casadesus G., Joseph J.A., and Perry G. (2002) Amyloid-beta and τ serve antioxidant functions in the aging and Alzheimer brain. Free Radical Biol. Med. 33, 1194–1999.
Lee H.G., Petersen R.B., Zhu X., et al. (2003) Will preventing protein aggregates live up to its promise as prophylaxis against neurodegenerative diseases? Brain Pathol. 13, 630–638.
Gomez-Isla T., Hollister R., West H., et al. (1997) Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann. Neurol. 41, 17–24.
Kril J.J., Patel S., Harding A.J., and Halliday G.M. (2002) Neuron loss from the hippocampus of Alzheimer’s disease exceeds extracellular neurofibrillary tangle formation. Acta Neuropathol. (Berl.) 103, 370–376.
Morsch R., Simon W., and Coleman P.D. (1999) Neurons may live for decades with neurofibrillary tangles. J. Neuropathol. Exp. Neurol. 58, 188–197.
Curtain C.C., Ali F., Volitakis I., et al. (2001) Alzheimer’s disease amyloid-beta binds copper and zinc to generate an allosterically ordered membrane-penetrating structure containing superoxide dismutase-like subunits. J. Biol. Chem. 276, 20,466–20,473.
Hock C., Golombowski S., Muller-Spahn F., et al. (1998) Cerebrospinal fluid levels of amyloid precursor protein and amyloid beta-peptide in Alzheimer’s disease and major depression—inverse correlation with dementia severity. Eur. Neurol. 39, 111–118.
Hou L., Kang I., Marchant R.E., and Zagorski M.G. (2002) Methionine 35 oxidation reduces fibril assembly of the amyloid abeta-(1–42) peptide of Alzheimer’s disease. J. Biol. Chem. 277, 40,173–40,176.
Zou K., Gong J.S., Yanagisawa K., and Michikawa M. (2002) A novel function of monomeric amyloid beta-protein serving as an antioxidant molecule against metal-induced oxidative damage. J. Neurosci. 22, 4833–4841.
Kontush A., Berndt C., Weber W., et al. (2001) Amyloid-beta is an antioxidant for lipoproteins in cerebrospinal fluid and plasma. Free Radical Biol. Med. 30, 119–128.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, X., Lee, Hg., Casadesus, G. et al. Oxidative imbalance in alzheimer’s disease. Mol Neurobiol 31, 205–217 (2005). https://doi.org/10.1385/MN:31:1-3:205
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/MN:31:1-3:205