Abstract
Background
Identification of a novel biomarker of subclinical lymph node metastasis (SLNM) in papillary thyroid microcarcinoma (PTMC) could provide important clues regarding SLNM in PTMC. We evaluated the significance of HGF and c-Met expression in surgically removed tumor tissue from PTMC patients as a predictive marker of SLNM.
Methods
We analyzed the immunohistochemical relationship between HGF and c-Met expression and SLNM in 113 surgically treated PTMC patients with clinically negative nodes presurgery. In addition, we explored whether HGF/c-Met pathway activation enhanced the in vitro migration and invasion of PTC cells.
Results
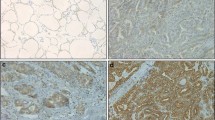
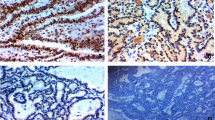
Positive immunohistochemical HGF and c-Met staining was found in 107 (95 %) and 103 (91 %) cases, respectively. The HGF staining distribution was as follows: no staining in 6 cases, weak staining in 43, moderate staining in 55, and strong staining in 9. Of the nine cases with strong HGF staining, eight (89 %) had SLNM. The c-Met staining distribution was as follows: no staining in 10 cases, weak staining in 39, moderate staining in 59, and strong staining in 5. Of the five cases with strong c-Met staining, three (60 %) had SLNM. The presence of SLNM was strongly correlated with HGF and c-Met expression in PTMC in a univariate analysis (P < 0.05). HGF overexpression was also associated with SLNM in a multivariate analysis (P < 0.05). Stimulation with exogenous HGF and constitutive activation of c-Met enhanced the migration and invasion of PTC cells in vitro by enhancing VEGF-A expression.
Conclusions
HGF/c-Met pathway activation is associated with SLNM of the central neck in PTMC.



Similar content being viewed by others
References
Hedinger C, William ED, Sobin LH. Histological typing of thyroid tumors. Vol 11. Berlin: Springer; 1988.
Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–7.
Pearce EN, Braverman LE. Papillary thyroid microcarcinoma outcomes and implications for treatment. J Clin Endocrinol Metab. 2004;89:3710–2.
Wada N, Duh QY, Sugino K, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg. 2003;237:399–407.
Lundgren CI, Hall P, Dickman PW, Zedenius J. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer. 2006;106:524–31.
So YK, Son YI, Hong SD, Seo MY, Baek CH, Jeong HS, Chung MK. Subclinical lymph node metastasis in papillary thyroid microcarcinoma: a study of 551 resections. Surgery. 2010;148:526–31.
Duffy MJ, McGowan PM, Gallagher WM. Cancer invasion and metastasis: changing views. J Pathol. 2008;214:283–93.
Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–25.
Jiang WG, Martin TA, Parr C, Davies G, Matsumoto K, Nakamura T. Hepatocyte growth factor, its receptor, and their potential value in cancer therapies. Crit Rev Oncol Hematol. 2005;53:35–69.
Matsumoto K, Nakamura T. Hepatocyte growth factor and the Met system as a mediator of tumor-stromal interactions. Int J Cancer. 2006;119:477–83.
Jiang WG, Davies G, Martin TA, Parr C, Watkins G, Mansel RE, Mason MD. The potential lymphangiogenic effects of hepatocyte growth factor/scatter factor in vitro and in vivo. Int J Mol Med. 2005;16:723–38.
Yücel OT, Sungur A, Kaya S. c-met overexpression in supraglottic laryngeal squamous cell carcinoma and its relation to lymph node metastases. Otolaryngol Head Neck Surg. 2004;130:698–703.
Ruggeri RM, Vitarelli E, Barresi G, Trimarchi F, Benvenga S, Trovato M. HGF/C-MET system pathways in benign and malignant histotypes of thyroid nodules: an immunohistochemical characterization. Histol Histopathol. 2012;27:113–21.
Jin L, Fuchs A, Schnitt SJ, et al. Expression of scatter factor and c-met receptor in benign and malignant breast tissue. Cancer. 1997;79:749–60.
Ramirez R, Hsu D, Patel A, Fenton C, Dinauer C, Tuttle RM, Francis GL. Over-expression of HGF and c-MET are associated with a high risk of metastasis and recurrence for children and young adults with papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2000;53:635–44.
Lloyd R, De Lellis R, Heitz P, Eng C. World Health Organization Classification of Tumours: pathology and genetics of tumours of the endocrine organs. Lyon: IARC Press; 2004.
Xie LQ, Bian LJ, Li Z, Li Y, Li ZX, Li B. Altered expression of E-cadherin by hepatocyte growth factor and effect on the prognosis of nasopharyngeal carcinoma. Ann Surg Oncol. 2010;17:1927–36.
Shaha AR, Tuttle RM, Shah JP. Papillary microcarcinoma of the thyroid. J Surg Oncol. 2007;95:532–3.
Shaha AR. Complications of neck dissection for thyroid cancer. Ann Surg Oncol. 2008;15:397–9.
Paccione RJ, Miyazaki H, Patel V, Waseem A, Gutkind JS, Zehner ZE, Yeudall WA. Keratin down-regulation in vimentin-positive cancer cells is reversible by vimentin RNA interference, which inhibits growth and motility. Mol Cancer Ther. 2008;7:2894–903.
Kim J, Takeuchi H, Lam ST, et al. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744–53.
Oler G, Camacho CP, Hojaij FC, Michaluart P Jr, Riggins GJ, Cerutti JM. Gene expression profiling of papillary thyroid carcinoma identifies transcripts correlated with BRAF mutational status and lymph node metastasis. Clin Cancer Res. 2008;14:4735–42.
Klein N, Picard E, Vignaud JM, et al. Vascular endothelial growth factor gene and protein: strong expression in thyroiditis and thyroid carcinoma. J Endocrinol. 1999;161:41–9.
Ide T, Kitajima Y, Miyoshi A, et al. Tumor-stromal cell interaction under hypoxia increases the invasiveness of pancreatic cancer cells through the hepatocyte growth factor/c-Met pathway. Int J Cancer. 2006;119:2750–9.
Miyata Y, Kanetake H, Kanda S. Presence of phosphorylated hepatocyte growth factor/c-Met is associated with tumor progression and survival in patients with conventional renal cell carcinoma. Clin Cancer Res. 2006;12:4876–81.
Murai M, Shen X, Huang L, et al. Overexpression of c-Met in oral SCC promotes hepatocyte growth factor-induced disruption of cadherin junctions and invasion. Int J Oncol. 2004;25:831–40.
Gentile A, Trusolino L, Comoglio PM. The met tyrosine kinase receptor in development and cancer. Cancer Metas Rev. 2008;27:85–94.
Chen BK, Ohtsuki Y, Furihata M, Takeuchi T, Iwata J, Liang SB, Sonobe H. Overexpression of c-Met protein in human thyroid tumors correlated with lymph node metastasis and clinicopathologic stage. Pathol Res Pract. 1999;195:427–33.
Ruco LP, Ranalli T, Marzullo A, Bianco P, Prat M, Comoglio PM, Baroni CD. Expression of Met protein in thyroid tumours. J Pathol. 1996;180:266–70.
Zhang YW, Su Y, Volpert OV, Vande Woude GF. Hepatocyte growth factor/scatter factor mediates angiogenesis through positive VEGF and negative thrombospondin 1 regulation. Proc Natl Acad Sci USA. 2003;100:12718–23.
Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEFG-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201:1089–99.
Hirakawa S. From tumor lymphangiogenesis to lymphvascular niche. Cancer Sci. 2009;100;983–9.
Mei L, Lin H, Ma S, Ji P, Xie D, Yu J. Vascular endothelial growth factor-A and -C: expression and correlations with lymphatic metastasis and prognosis in colorectal cancer. Med Oncol. 2011;28:151–8.
Nagy JA, Vasile E, Feng D, et al. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med. 2002;196:1497–506.
Acknowledgment
This work was supported by a Konkuk University Medical Center Research Grant 2011.
Disclosures
The authors indicated no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Fig. S1
Exogenous HGF stimulates the migration and invasion of PTC cells in vitro. a Invasion assay results. Transwell chambers were used to assess cell invasiveness. TPC-1 cells were seeded on the upper membrane in the presence of recombinant human HGF (0, 10, or 30 ng/ml). After incubation for 48 h, the attached cells in the lower section of the upper chamber were counted by light microscopy. b Wound healing assay results. TPC1 cells were plated in culture plates at a density of ~1 × 105/well and grown to confluency. The monolayer was scratched with a pipette tip and washed with phosphate-buffered saline. To evaluate the effect of HGF on migratory activities, TPC-1 cells were treated with HGF (0, 10, or 30 ng/ml). Wound healing was documented by photography 24 h after scratching. c Effect of HGF on the expression of VEGF. TPC-1 cells were treated with 30 ng/ml HGF for the indicated lengths of time. Then, RNA was extracted and the expression of VEGF family members was evaluated by RT-PCR
Fig. S2
Constitutive activation of c-Met promotes invasion of PTC cells in vitro. a Western blot analysis of ligand-independent, constitutive activation of c-Met. c-Met, full-length c-Met (130 kDa); p-Met, phospho-c-Met (68 kDa). b Invasion assay results. TPC-c-Met and TPC-control cells were seeded in the upper chamber. After incubation for 48 h, invading cells attached to the lower surface of the membrane were counted. c Wound healing assay. TPC-c-Met and TPC-control cells were plated in culture plates at a density of ~1 × 105/well and grown to confluency. The monolayer was scratched with a pipette tip and washed with phosphate-buffered saline. Wound healing was documented by photography 24 h after scratching. d Effect of c-Met signaling activation on VEGF expression. TPC-c-Met and TPC-control cells were cultured in RPMI medium supplemented with 10 % FBS. Then, RNA was extracted and the expression of various VEGF family members was evaluated by semi-quantitative RT-PCR. In addition, VEGF-A protein levels was studied by Western blotting in these cells. e Effect of bevacizumab on the induction of TPC cell invasion by constitutive c-Met activation. BCM, 300 ng/ml bevacizumab, *P<0.05. f Effect of bevacizumab on the stimulation of TPC cell migration by constitutive c-Met activation. BCM, 300 ng/ml bevacizumab
Rights and permissions
About this article
Cite this article
Koo, B.S., Kim, J.M., Seo, S.T. et al. Upregulation of HGF and c-MET is Associated with Subclinical Central Lymph Node Metastasis in Papillary Thyroid Microcarcinoma. Ann Surg Oncol 21, 2310–2317 (2014). https://doi.org/10.1245/s10434-014-3553-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-3553-5