Abstract
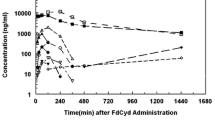
In vivo, the DNA methyltransferase inhibitor, 5-fluoro-2′-deoxycytidine (FdCyd, NSC-48006), is rapidly converted to its unwanted metabolites. Tetrahydrouridine (THU, NSC-112907), a cytidine deaminase inhibitor can block the first metabolic step in FdCyd catabolism. Clinical studies have shown that co-administration with THU can inhibit the metabolism of FdCyd. The National Cancer Institute is particularly interested in a 1:5 FdCyd/THU formulation. The purpose of this study was to investigate the in vitro pH stability of FdCyd and THU individually and in combination. A stability-indicating high-performance liquid chromatography method for the quantification of both compounds and their degradants was developed using a ZIC®-HILIC column. The effect of THU and FdCyd on the in vitro degradation of each other was studied as a function of pH from 1.0 to 7.4 in aqueous solutions at 37°C. The degradation of FdCyd appears to be first-order and acid-catalyzed. THU equilibrates with at least one of its degradants. The combination of FdCyd and THU in solution does not affect the stability of either compound. The stability and compatibility of FdCyd and THU in the solid state at increased relative humidity and at various temperatures are also evaluated.










Similar content being viewed by others
References
Beumer JH, Eiseman JL, Parise RA, Joseph E, Holleran JL, Covey JM, et al. Pharmacokinetics, metabolism, and oral bioavailability of the DNA methyltransferase inhibitor 5-fluoro-2′-deoxycytidine in mice. Clin Cancer Res. 2006;12:7483–91.
Beumer JH, Parise RA, Newman EM, Doroshow JH, Synold TW, Lenz H-J, et al. Concentrations of the DNA methyltransferase inhibitor 5-fluoro-2′-deoxycytidine (FdCyd) and its cytotoxic metabolites in plasma of patients treated with FdCyd and tetrahydrouridine (THU). Cancer Chemother Pharmacol. 2008;62:363–8.
Stoller RG, Myers CE, Chabner BA. Analysis of cytidine deaminase and tetrahydrouridine interaction by use of ligand techniques. Biochem Pharmacol. 1978;27:53–9.
Boothman DA, Briggle TV, Greer S. Tumor-selective metabolism of 5-fluoro-2′-deoxycytidine coadministered with tetrahydrouridine compared to 5-fluorouracil in mice bearing Lewis lung carcinoma. Cancer Res. 1987;47:2354–62.
Xiang T, Niemi R, Bummer P, Anderson BD. Epimer interconversion, isomerization, and hydrolysis of tetrahydrouridine: implications for cytidine deaminase inhibition. J Pharma Sci. 2003;92:2027–39.
Acknowledgments
This work was supported by National Cancer Institute under the contract N01-CM-27142.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, D., Myrdal, P.B., Karlage, K.L. et al. Stability of 5-Fluoro-2′-deoxycytidine and Tetrahydrouridine in Combination. AAPS PharmSciTech 11, 247–252 (2010). https://doi.org/10.1208/s12249-010-9383-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-010-9383-2