Abstract
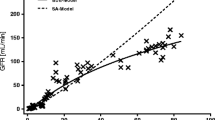
A model was developed that characterized the maturation and growth of the renal function parameters (RFPs) glomerular filtration rate (GF), active tubular secretion (AS), and renal plasma flow (QR). Published RFP values were obtained from 63 healthy children between the ages of 2 days and 12 years. Maturation over time was assumed to be exponential from an immature (RFPim) to a mature (RFPma) level; for growth, RFPim and RFPma were assumed to follow the allometric equation: RFP(age, W)=aWbe−kmat *age+cWb(1−e−kmat *age), where W is body weight, kmat is the maturation rate constant, b is the body weight exponent, and a and c are RFPim and RFPma at unit W. The model-based equation was fitted to the age-W, RFP values by a nonlinear least-squares method. For GF, the maturation half-life was 7.9 months (90% maturation, 26 months), the body weight exponent was 0.662, and the ratio c/a (which reflected the magnitude of the maturation influence) was 3.1. For AS and QR, the maturation half-lives were about 3.8 months and the ratio c/a was about 1.8. For renally eliminated drugs, the model can be used to estimate dosing regimens that are based on the adult dosing regimen and the age and weight of the child.
Similar content being viewed by others
References
Rowland M, Tozer T. Clinical Pharmacokinetics, Concepts and Applications. 3rd ed. Baltimore: Williams and Wilkins, 1995:83–105.
Benet L, Oie S, Schwartz J. Appendix II: Design and optimization of dosage regimens; pharmacokinetic data. In: Hardman J, Limbird L, Molinoff P, Ruddon R, Gilman A, eds. Goodman And Gilmans The Pharmacological Basis Of Therapeutics. 9th ed. New York: McGraw-Hill, 1996:1707–1792.
Crom WR. Pharmacokinetics in the child. Environ Health Perspect. 1994;102:111–117.
Rowland M, Tozer T. Clinical Pharmacokinetics, Concepts and Applications. 3rd ed. Baltimore: Williams and Wilkins, 1995:230–247.
Lam YWF, Banerji S, Hatfield C, Talbert RL. Principles of drug administration in renal insufficiency. Clin Pharmacokinet. 1997;32:30–57.
Hayton W, Kneer J, De Groot R, Stoeckel K. Influence of maturation and growth on cefetamet pivoxil pharmacokinetics: rational dosing for infants. Antimicrob Agents Chemother. 1996;40:567–574.
Rubin M, Bruck E, Rapoport M. Maturation of renal function in childhood: clearance studies. J Clin Invest. 1949;28:1144–1162.
Schmidt-Nielsen K. Scaling. Why is Animal Size So important? Cambridge: Cambridge University Press; 1984:14–32.
Hallynck T, Soep H, Thomis J, Boelaert J, Daneels R, Dettli L. Should clearance be normalised to body surface or to lean body mass? Br J Pharmacol. 1981;11:523–526.
Heilbron DC, Holliday MA, Al-Dahwi A, Kogan BA. Expressing glomerular filtration rate in children. Pediatr Nephrol. 1991;5:5–11.
Anderson B, McKee A, Holford N. Size, myths and the clinical pharmacokinetics of analgesia in paediatric patients. Clin Pharmacokinet. 1997;33:313–327.
Diem K, Lentner C, eds. Scientific Tables. Basie, Switzerland: Ciba-Geigy Ltd., 1970:693–705.
Kauffman, RE. Status of drug approval processes and regulation of medications for children. Curr Opin Pediatr. 1995;7:195–198.
Anon. Pediatric rule. AAPS News. 1999; February:4–5.
Rowland M, Tozer T. Clinical Pharmacokinetics, Concepts and Applications. 3rd ed. Baltimore: Williams and Wilkins, 1995:254–255.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published: March 3, 2000
Rights and permissions
About this article
Cite this article
Hayton, W.L. Maturation and growth of renal function: Dosing renally cleared drugs in children. AAPS PharmSci 2, 3 (2000). https://doi.org/10.1208/ps020103
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/ps020103