Abstract
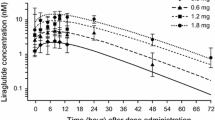
A potent novel compound (MK-3577) was developed for the treatment of type 2 diabetes mellitus (T2DM) through blocking the glucagon receptor. A semi-mechanistic model was developed to describe the drug effect on glucagon and the interaction between glucagon, insulin, and glucose in healthy subjects (N = 36) during a glucagon challenge study in which glucagon, octreotide (Sandostatin), and basal insulin were infused for 2 h starting from 3, 12, or 24 h postdose of a single 0–900 mg MK-3577 administration. The drug effect was modeled by using an inhibitory E max model (I max = 0.96 and IC50 = 13.9 nM) to reduce the ability of glucagon to increase the glucose production rate (GPROD). In addition, an E max model (E max = 0.79 and EC50 = 575 nM) to increase glucagon secretion by the drug was used to account for the increased glucagon concentrations prechallenge (via compensatory feedback). The model adequately captured the observed profiles of glucagon, glucose, and insulin pre- and postchallenge. The model was then adapted for the T2DM patient population. A linear model to correlate fasting plasma glucose (FPG) to weighted mean glucose (WMG) was developed and provided robust predictions to assist with the dose adjustment for the interim analysis of a phase IIa study.




Similar content being viewed by others
REFERENCES
Porte Jr D. Beta-cells in type II diabetes mellitus. Diabetes. 1991;40:166–80.
Aronoff SL, Berkowitz K, Shreiner B, Want L. Glucose metabolism and regulation: beyond insulin and glucagon. Diabetes Spectr. 2004;17:183–90.
Rizza RA, Cryer PE, Haymond MW, Gerich JE. Adrenergic mechanisms for the effects of epinephrine on glucose production and clearance in man. J Clin Invest. 1980;65:682–9.
Cheng D. Prevalence, predisposition and prevention of type II diabetes. Nutr Metab. 2005;2:29.
Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
Li XC, Zhuo JL. Targeting glucagon receptor signaling in treating metabolic syndrome and renal injury in type 2 diabetes: theory versus promise. Clin Sci (Lond). 2007;113(4):183–93.
Hare KJ. Role of GLP-1 induced glucagon suppression in type 2 diabetes mellitus. Dan Med Bull. 2010;57:B4181.
Del Guercio MJ, di Natale B, Gargantini L, Garlaschi C, Chiumello G. Effect of somatostatin on blood sugar, plasma growth hormone, and glucagon levels in diabetic children. Diabetes. 1976;25:550–3.
Silber HE, Jauslin PM, Frey N, Gieschke R, Simonsson USH, Karlsson MO. An integrated model for glucose and insulin regulation in healthy volunteers and type 2 diabetic patients following intravenous glucose provocations. J Clin Pharmacol. 2007;47:1159–71.
Jauslin PM, Silber HE, Frey N, Gieschke R, Simonsson USH, Jorga K, et al. An integrated glucose-insulin model to describe oral glucose tolerance test data in type 2 diabetics. J Clin Pharmacol. 2007;47:1244–55.
Man CD, Rizza RA, Cobelli C. Meal simulation model of the glucose-insulin system. IEEE Trans Biomed Eng. 2007;54:1740–9.
de Winter W, DeJongh J, Post T, Ploeger B, Urquhart R, Moules I, et al. A mechanism-based disease progression model for comparison of long-term effects of pioglitazone, metformin and gliclazide on disease processes underlying type 2 diabetes mellitus. J Pharmacokinet Pharmacodyn. 2006;33:313–43.
Hovorka R, Canonico V, Chassin LJ, Haueter U, Massi-Benedetti M, Federici MO, et al. Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas. 2004;25:905–20.
Lau YY, Ma P, Gibiansky L, Komorowski R, Wang J, Wang G, et al. Pharmacokinetic and pharmacodynamic modeling of a monoclonal antibody antagonist of glucagon receptor in male ob/ob mice. AAPS J. 2009;11:700–9.
Schneck KB, Zhang X, Bauer R, Karlsson MO, Sinha VP. Assessment of glycemic response to an oral glucokinase activator in a proof of concept study: application of a semi-mechanistic, integrated glucose-insulin-glucagon model. J Pharmacokinet Pharmacodyn. 2013;40:67–80.
Sorensen JT. A physiologic model of glucose metabolism in man and its use to design and assess improved insulin therapies for diabetes. Thesis (Sc. D.), Massachusetts Institute of Technology, Dept. of Chemical Engineering; 1985. http://dspace.mit.edu/handle/1721.1/15234.
Schaller S, Willmann S, Lippert J, Schaupp L, Pieber TR, Schuppert A, et al. A generic integrated physiologically based whole-body model of the glucose-insulin-glucagon regulatory system. CPT Pharmacometrics Syst Pharmacol. 2013;2:e65.
Harris AG. Somatostatin and somatostatin analogues: pharmacokinetics and pharmacodynamic effects. Gut. 1994;35:S1–4. doi:10.1136/gut.35.3_Suppl.S1.
Chanson P, Timsit J, Harris AG. Clinical pharmacokinetics of octreotide: therapeutic applications in patients with pituitary tumors. Clin Pharmacokinet. 1993;25:375–91.
Holford N, Ma SC, Ploeger BA. Clinical trial simulation: a review. Nature. 2010;88:166–82.
Sloop KW, Cao JX, Siesky AM, Zhang HY, Bodenmiller DM, Cox AL, et al. Hepatic and glucagon-like peptide-1-mediated reversal of diabetes by glucagon receptor antisense oligonucleotide inhibitors. J Clin Invest. 2004;113:1571–81.
Engel SS, Reitman ML, Xu L, Andryuk PJ, Davies MJ, Kaufman KD, et al. Glycemic and lipid effects of the short-acting glucagon receptor antagonist MK-3577 in patients with type 2 diabetes. Presentation #1037-P, ADA 72nd Scientific Sessions. 2012.
Rohlfing CL, England JD, Tennill A, Little RR, Goldstein DE. Defining the relationship between plasma glucose and HbA1c. Diabetes Care. 2002;25(2):275–8.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Marcella K. Ruddy for contributions to the glucagon study design and Merck & Co., Inc. provided the funding source.
Financial Disclosure
Joanna Z. Peng, William S. Denney, Bret J. Musser, Rong Liu, Kuenhi Tsai, Lanyan Fang, Marc L. Reitman, Matthew D. Troyer, Samuel S. Engel, Lei Xu, Aubrey Stoch, and Julie A. Stone were employees of Merck & Co., Inc. when the work for this article was conducted and may own stock or hold stock options. Ken G. Kowalski is a consultant for Merck & Co., Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
The corresponding author was a Merck employee when this work was conducted.
Rights and permissions
About this article
Cite this article
Peng, J.Z., Denney, W.S., Musser, B.J. et al. A Semi-mechanistic Model for the Effects of a Novel Glucagon Receptor Antagonist on Glucagon and the Interaction Between Glucose, Glucagon, and Insulin Applied to Adaptive Phase II Design. AAPS J 16, 1259–1270 (2014). https://doi.org/10.1208/s12248-014-9648-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-014-9648-x