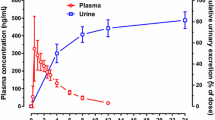
Abstract
Lenalidomide is a synthetic derivative of thalidomide exhibiting multiple immunomodulatory activities beneficial in the treatment of several hematological malignancies. Murine pharmacokinetic characterization necessary for translational and further preclinical investigations has not been published. Studies herein define mouse plasma pharmacokinetics and tissue distribution after intravenous (IV) bolus administration and bioavailability after oral and intraperitoneal delivery. Range finding studies used lenalidomide concentrations up to 15 mg/kg IV, 22.5 mg/kg intraperitoneal injections (IP), and 45 mg/kg oral gavage (PO). Pharmacokinetic studies evaluated doses of 0.5, 1.5, 5, and 10 mg/kg IV and 0.5 and 10 mg/kg doses for IP and oral routes. Liquid chromatography–tandem mass spectrometry was used to quantify lenalidomide in plasma, brain, lung, liver, heart, kidney, spleen, and muscle. Pharmacokinetic parameters were estimated using noncompartmental and compartmental methods. Doses of 15 mg/kg IV, 22.5 mg/kg IP, and 45 mg/kg PO lenalidomide caused no observable toxicity up to 24 h postdose. We observed dose-dependent kinetics over the evaluated dosing range. Administration of 0.5 and 10 mg/kg resulted in systemic bioavailability ranges of 90–105% and 60–75% via IP and oral routes, respectively. Lenalidomide was detectable in the brain only after IV dosing of 5 and 10 mg/kg. Dose-dependent distribution was also observed in some tissues. High oral bioavailability of lenalidomide in mice is consistent with oral bioavailability in humans. Atypical lenalidomide tissue distribution was observed in spleen and brain. The observed dose-dependent pharmacokinetics should be taken into consideration in translational and preclinical mouse studies.




Similar content being viewed by others
References
Muller GW, Chen R, Huang SY, Corral LG, Wong LM, Patterson RT, et al. Amino-substituted thalidomide analogs: potent inhibitors of TNF-alpha production. Bioorg Med Chem Lett. 1999;9(11):1625–30. Epub 1999/07/01.
Dredge K, Marriott JB, Macdonald CD, Man HW, Chen R, Muller GW, et al. Novel thalidomide analogues display anti-angiogenic activity independently of immunomodulatory effects. Br J Cancer. 2002;87(10):1166–72. Epub 2002/10/29.
Dredge K, Horsfall R, Robinson SP, Zhang LH, Lu L, Tang Y, et al. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvasc Res. 2005;69(1-2):56–63. Epub 2005/03/31.
Moutouh-de Parseval LA, Verhelle D, Glezer E, Jensen-Pergakes K, Ferguson GD, Corral LG, et al. Pomalidomide and lenalidomide regulate erythropoiesis and fetal hemoglobin production in human CD34+ cells. J Clin Investig. 2008;118(1):248–58. Epub 2007/12/08.
Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98(1):210–6. Epub 2001/06/22.
Corral LG, Haslett PA, Muller GW, Chen R, Wong LM, Ocampo CJ, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163(1):380–6. Epub 1999/06/29.
Wu L, Adams M, Carter T, Chen R, Muller G, Stirling D, et al. lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clinical cancer research. Off J Am Assoc Cancer Res. 2008;14(14):4650–7. Epub 2008/07/17.
Zhu D, Corral LG, Fleming YW, Stein B. Immunomodulatory drugs Revlimid (lenalidomide) and CC-4047 induce apoptosis of both hematological and solid tumor cells through NK cell activation. Cancer Immunol Immunother: CII. 2008;57(12):1849–59. Epub 2008/04/09.
Lu L, Payvandi F, Wu L, Zhang LH, Hariri RJ, Man HW, et al. The anti-cancer drug lenalidomide inhibits angiogenesis and metastasis via multiple inhibitory effects on endothelial cell function in normoxic and hypoxic conditions. Microvasc Res. 2009;77(2):78–86.
Gandhi AK, Kang J, Capone L, Parton A, Wu L, Zhang LH, et al. Dexamethasone synergizes with lenalidomide to inhibit multiple myeloma tumor growth, but reduces lenalidomide-induced immunomodulation of T and NK cell function. Curr Cancer Drug Targets. 2010;10(2):155–67. Epub 2010/01/22.
Richardson PG, Schlossman RL, Weller E, Hideshima T, Mitsiades C, Davies F, et al. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood. 2002;100(9):3063–7. Epub 2002/10/18.
Sanchorawala V, Wright DG, Rosenzweig M, Finn KT, Fennessey S, Zeldis JB, et al. Lenalidomide and dexamethasone in the treatment of AL amyloidosis: results of a phase 2 trial. Blood. 2007;109(2):492–6. Epub 2006/09/09.
Dispenzieri A, Lacy MQ, Zeldenrust SR, Hayman SR, Kumar SK, Geyer SM, et al. The activity of lenalidomide with or without dexamethasone in patients with primary systemic amyloidosis. Blood. 2007;109(2):465–70. Epub 2006/09/30.
Chanan-Khan A, Miller KC, Musial L, Lawrence D, Padmanabhan S, Takeshita K, et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24(34):5343–9. Epub 2006/11/08.
Chanan-Khan AA, Cheson BD. Lenalidomide for the treatment of B-cell malignancies. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26(9):1544–52. Epub 2008/02/21.
Ferrajoli A, Lee BN, Schlette EJ, O'Brien SM, Gao H, Wen S, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111(11):5291–7.
Habermann TM, Lossos IS, Justice G, Vose JM, Wiernik PH, McBride K, et al. Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. Br J Haematol. 2009;145(3):344–9. Epub 2009/02/28.
Tefferi A, Cortes J, Verstovsek S, Mesa RA, Thomas D, Lasho TL, et al. Lenalidomide therapy in myelofibrosis with myeloid metaplasia. Blood. 2006;108(4):1158–64. Epub 2006/04/13.
Witzig TE, Wiernik PH, Moore T, Reeder C, Cole C, Justice G, et al. Lenalidomide oral monotherapy produces durable responses in relapsed or refractory indolent non-Hodgkin's Lymphoma. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(32):5404–9. Epub 2009/10/07.
Wiernik PH, Lossos IS, Tuscano JM, Justice G, Vose JM, Cole CE, et al. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin's lymphoma. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26(30):4952–7. Epub 2008/07/09.
Chen N, Wen L, Lau H, Surapaneni S, Kumar G. Pharmacokinetics, metabolism and excretion of [(14)C]-lenalidomide following oral administration in healthy male subjects. Cancer Chemotherapy and Pharmacology. 2012;69(3):789–97.
Revlimid Label: REVLIMID® (lenalidomide) 5 mg, 10 mg, 15 mg and 25 mg capsules. 2009.
Hofmeister CC, Yang X, Pichiorri F, Chen P, Rozewski DM, Johnson AJ, et al. Phase I trial of lenalidomide and CCI-779 in patients with relapsed multiple myeloma: evidence for lenalidomide-CCI-779 interaction via P-glycoprotein. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29(25):3427–34. Epub 2011/08/10.
Chen N, Lau H, Kong L, Kumar G, Zeldis JB, Knight R, et al. Pharmacokinetics of lenalidomide in subjects with various degrees of renal impairment and in subjects on hemodialysis. J Clin Pharmacol. 2007;47(12):1466–75.
Chen N, Lau H, Choudhury S, Wang X, Assaf M, Laskin OL. Distribution of lenalidomide into semen of healthy men after multiple oral doses. J Clin Pharmacol. 2010;50(7):767–74. Epub 2010/02/18.
Fine HA, Kim L, Albert PS, Duic JP, Ma H, Zhang W, et al. A phase I trial of lenalidomide in patients with recurrent primary central nervous system tumors. Clin Cancer Res. 2007;13(23):7101–6. Epub 2007/12/07.
Dahut WL, Aragon-Ching JB, Woo S, Tohnya TM, Gulley JL, Arlen PM, et al. Phase I study of oral lenalidomide in patients with refractory metastatic cancer. J Clin Pharmacol. 2009;49(6):650–60. Epub 2009/05/20.
Iida S, Chou T, Okamoto S, Nagai H, Hatake K, Murakami H, et al. Lenalidomide plus dexamethasone treatment in Japanese patients with relapsed/refractory multiple myeloma. Int J Hematol. 2010;92(1):118–26. Epub 2010/06/19.
Muscal JA, Sun Y, Nuchtern JG, Dauser RC, McGuffey LH, Gibson BW, et al. Plasma and cerebrospinal fluid pharmacokinetics of thalidomide and lenalidomide in nonhuman primates. Cancer Chemother Pharmacol. 2011. Epub 2011/11/24.
Richardson PG, Blood E, Mitsiades CS, Jagannath S, Zeldenrust SR, Alsina M, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108(10):3458–64. Epub 2006/07/15.
Rajkumar SV, Hayman SR, Lacy MQ, Dispenzieri A, Geyer SM, Kabat B, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106(13):4050–3. Epub 2005/08/25.
Zonder JA, Barlogie B, Durie BG, McCoy J, Crowley J, Hussein MA. Thrombotic complications in patients with newly diagnosed multiple myeloma treated with lenalidomide and dexamethasone: benefit of aspirin prophylaxis. Blood. 2006;108(1):403. author reply 4. Epub 2006/06/23.
Palumbo A, Rus C, Zeldis JB, Rodeghiero F, Boccadoro M. Enoxaparin or aspirin for the prevention of recurrent thromboembolism in newly diagnosed myeloma patients treated with melphalan and prednisone plus thalidomide or lenalidomide. J Thromb Haemost. 2006;4(8):1842–5. Epub 2006/08/02.
Andritsos LA, Johnson AJ, Lozanski G, Blum W, Kefauver C, Awan F, et al. Higher doses of lenalidomide are associated with unacceptable toxicity including life-threatening tumor flare in patients with chronic lymphocytic leukemia. J Clin Oncol. 2008;26(15):2519–25. Epub 2008/04/23.
Blum W, Klisovic RB, Becker H, Yang X, Rozewski DM, Phelps MA, et al. Dose escalation of lenalidomide in relapsed or refractory acute leukemias. J Clin Oncol. 2010;28(33):4919–25. Epub 2010/10/20.
Warren KE, Goldman S, Pollack IF, Fangusaro J, Schaiquevich P, Stewart CF, et al. Phase I trial of lenalidomide in pediatric patients with recurrent, refractory, or progressive primary CNS tumors: Pediatric Brain Tumor Consortium study PBTC-018. J Clin Oncol. 2011;29(3):324–9. Epub 2010/12/15.
Qian Z, Zhang L, Cai Z, Sun L, Wang H, Yi Q, et al. Lenalidomide synergizes with dexamethasone to induce growth arrest and apoptosis of mantle cell lymphoma cells in vitro and in vivo. Leuk Res. 2011;35(3):380–6. Epub 2010/11/05.
Santo L, Hideshima T, Cirstea D, Bandi M, Nelson EA, Gorgun G, et al. Antimyeloma activity of a multitargeted kinase inhibitor, AT9283, via potent Aurora kinase and STAT3 inhibition either alone or in combination with lenalidomide. Clin Cancer Res: Off J Am Assoc Cancer Res. 2011;17(10):3259–71. Epub 2011/03/25.
Schmidt M, Kim Y, Gast SM, Endo T, Lu D, Carson D, et al. Increased in vivo efficacy of lenalidomide and thalidomide by addition of ethacrynic acid. In vivo. 2011;25(3):325–33.
Benson Jr DM, Bakan CE, Zhang S, Collins SM, Liang J, Srivastava S, et al. IPH2101, a novel anti-inhibitory KIR antibody, and lenalidomide combine to enhance the natural killer cell versus multiple myeloma effect. Blood. 2011;118(24):6387–91. Epub 2011/10/28.
Chauhan D, Singh AV, Ciccarelli B, Richardson PG, Palladino MA, Anderson KC. Combination of novel proteasome inhibitor NPI-0052 and lenalidomide trigger in vitro and in vivo synergistic cytotoxicity in multiple myeloma. Blood. 2010;115(4):834–45. Epub 2009/12/08.
Galustian C, Meyer B, Labarthe MC, Dredge K, Klaschka D, Henry J, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother: CII. 2009;58(7):1033–45. Epub 2008/11/15.
Yang XX, Hu ZP, Xu AL, Duan W, Zhu YZ, Huang M, et al. A mechanistic study on reduced toxicity of irinotecan by coadministered thalidomide, a tumor necrosis factor-alpha inhibitor. J Pharmacol Exp Ther. 2006;319(1):82–104. Epub 2006/07/04.
Kenyon BM, Browne F, D'Amato RJ. Effects of thalidomide and related metabolites in a mouse corneal model of neovascularization. Exp Eye Res. 1997;64(6):971–8. Epub 1997/06/01.
Meyring M, Muhlbacher J, Messer K, Kastner-Pustet N, Bringmann G, Mannschreck A, et al. In vitro biotransformation of (R)- and (S)-thalidomide: application of circular dichroism spectroscopy to the stereochemical characterization of the hydroxylated metabolites. Anal Chem. 2002;74(15):3726–35. Epub 2002/08/15.
Physiological Parameter Values for PBPK Models. [database on the Internet]. International Life Sciences Institute and Risk Science Institute. 1994.
Gjedde SB, Gjeode A. Organ blood flow rates and cardiac output of the BALB/c mouse. Comp Biochem Physiol Part A: Physiol. 1980;67(4):671–4.
Berg SL, Cairo MS, Russell H, Ayello J, Ingle AM, Lau H, et al. Safety, pharmacokinetics, and immunomodulatory effects of lenalidomide in children and adolescents with relapsed/refractory solid tumors or myelodysplastic syndrome: a Children's Oncology Group Phase I Consortium report. J Clin Oncol: Off J Am Soc Clin Oncol. 2011;29(3):316–23. Epub 2010/12/15.
Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J: Off Publ Fed Am Soc Exp Biol. 2008;22(3):659–61. Epub 2007/10/19.
Liu WM, Laux H, Henry JY, Bolton TB, Dalgleish AG, Galustian C. A microarray study of altered gene expression in colorectal cancer cells after treatment with immunomodulatory drugs: differences in action in vivo and in vitro. Mol Biol Rep. 2010;37(4):1801–14. Epub 2009/07/15.
Kiaei M, Petri S, Kipiani K, Gardian G, Choi DK, Chen J, et al. Thalidomide and lenalidomide extend survival in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci: Off J Soc Neurosci. 2006;26(9):2467–73. Epub 2006/03/03.
Neymotin A, Petri S, Calingasan NY, Wille E, Schafer P, Stewart C, et al. Lenalidomide (Revlimid) administration at symptom onset is neuroprotective in a mouse model of amyotrophic lateral sclerosis. Exp Neurol. 2009;220(1):191–7. Epub 2009/09/08.
NCBI. PubChem Compound. NCBI; 2012 [cited 2012 March 21].
Baird R, van Zyl-Smit RN, Iveson A, Duddy J, Rassam SM. Thalidomide is highly effective in a patient with meningeal acute myeloid leukaemia. Leuk Lymphoma. 2004;45(1):179–81. Epub 2004/04/06.
Hwu WJ, Raizer J, Panageas KS, Lis E. Treatment of metastatic melanoma in the brain with temozolomide and thalidomide. Lancet Oncol. 2001;2(10):634–5. Epub 2002/03/21.
Loscher W, Potschka H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. Neuro Rx. 2005;2(1):86–98. Epub 2005/02/18.
Sharma RA, Steward WP, Daines CA, Knight RD, O'Byrne KJ, Dalgleish AG. Toxicity profile of the immunomodulatory thalidomide analogue, lenalidomide: phase I clinical trial of three dosing schedules in patients with solid malignancies. Eur J Cancer. 2006;42(14):2318–25. Epub 2006/08/11.
Rao KV. Lenalidomide in the treatment of multiple myeloma. Am J Health Syst Pharm. 2007;64(17):1799–807.
Acknowledgments
This work was supported by an Eli Lilly Graduate Fellowship (DMR), a Leukemia and Lymphoma Society SCOR grant (JCB, AJJ, and MP), the D. Warren Brown Foundation (JCB), and NIH grants 1 P50 CA140158 (JCB and AJJ), K12CA133250 (AJJ), and 5KL2RR025754.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Rozewski, D.M., Herman, S.E.M., Towns, W.H. et al. Pharmacokinetics and Tissue Disposition of Lenalidomide in Mice. AAPS J 14, 872–882 (2012). https://doi.org/10.1208/s12248-012-9401-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-012-9401-2