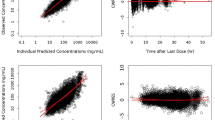
Abstract
The objective of this work was to examine the atazanavir–bilirubin relationship using a population-based approach and to assess the possible application of bilirubin as a readily available marker of atazanavir exposure. A model of atazanavir exposure and its concentration-dependent effect on bilirubin levels was developed based on 200 atazanavir and 361 bilirubin samples from 82 patients receiving atazanavir in the NORTHIV trial. The pharmacokinetics was adequately described by a one-compartment model with first-order absorption and lag-time. The maximum inhibition of bilirubin elimination rate constant (I max) was estimated at 91% (95% CI, 87–94) and the atazanavir concentration resulting in half of I max (IC50) was 0.30 μmol/L (95% CI, 0.24–0.37). At an atazanavir/ritonavir dose of 300/100 mg given once daily, the bilirubin half-life was on average increased from 1.6 to 8.1 h. A nomogram, which can be used to indicate suboptimal atazanavir exposure and non-adherence, was constructed based on model simulations.





Similar content being viewed by others
References
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. January 10, 2011; 1–166. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed January 2011.
Josephson F, Albert J, Flamholc L, Gisslén M, Karlström O, Moberg L, et al. Treatment of HIV infection: Swedish recommendations 2009. Scand J Infect Dis. 2009;41(11–12):788–807.
Zhang D, Chando T, Everett D, Patten C, Dehal S, Humphreys W. In vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationship of this property to in vivo bilirubin glucuronidation. Drug Metabolism and Disposition. 2005;33(11):1729.
Smith D, Jeganathan S, Ray J. Atazanavir plasma concentrations vary significantly between patients and correlate with increased serum bilirubin concentrations. HIV Clinical Trials. 2006;7(1):34–8.
Cleijsen R, Van de Ende M, Kroon F, Lunel F, Koopmans P, Gras L, et al. Therapeutic drug monitoring of the HIV protease inhibitor atazanavir in clinical practice. J Antimicrob Chemother. 2007;60(4):897.
Rodríguez-Nóvoa S, Martín-Carbonero L, Barreiro P, González-Pardo G, Jiménez-Nácher I, González-Lahoz J, et al. Genetic factors influencing atazanavir plasma concentrations and the risk of severe hyperbilirubinemia. AIDS. 2007;21(1):41.
Monaghan G, Ryan M, Hume R, Burchell B, Seddon R. Genetic variation in bilirubin UDP-glucuronosyltransferase gene promoter and Gilbert’s syndrome. Lancet. 1996;347(9001):578–81.
Annaert P, Ye Z, Stieger B, Augustijns P. Interaction of HIV protease inhibitors with OATP1B1, 1B3, and 2B1. Xenobiotica. 2010;40(3):163–76.
Cui Y, König J, Leier I, Buchholz U, Keppler D. Hepatic uptake of bilirubin and its conjugates by the human organic anion transporter SLC21A6. J Biol Chem. 2001;276(13):9626.
Mediavilla M, Pascolo L, Rodriguez J, Guibert E, Ostrow J, Tiribelli C. Uptake of [3H] bilirubin in freshly isolated rat hepatocytes: role of free bilirubin concentration. FEBS Lett. 1999;463(1–2):143–5.
Hartkoorn R, Kwan W, Shallcross V, Chaikan A, Liptrott N, Egan D, et al. HIV protease inhibitors are substrates for OATP1A2, OATP1B1 and OATP1B3 and lopinavir plasma concentrations are influenced by SLCO1B1 polymorphisms. Pharmacogenetics and Genomics. 2010;20(2):112.
Campbell S, de Morais S, Xu J. Inhibition of human organic anion transporting polypeptide OATP 1B1 as a mechanism of drug-induced hyperbilirubinemia. Chemico–Biological Interactions. 2004;150(2):179–87.
Perloff ES, Duan SX, Skolnik PR, Greenblatt DJ, von Moltke LL. Atazanavir: effects on P-glycoprotein transport and CYP3A metabolism in vitro. Drug Metabolism and Disposition. 2005;33(6):764.
Busti AJ, Hall RG, Margolis DM. Atazanavir for the treatment of human immunodeficiency virus infection. Pharmacotherapy. 2004;24(12):1732–47.
Rodriguez Novoa S, Barreiro P, Rendón A, Barrios A, Corral A, Jimenez-Nacher I. Plasma levels of atazanavir and the risk of hyperbilirubinemia are predicted by the 3435C T polymorphism at the multidrug resistance gene 1. Clinical Infectious Diseases. 2006;42(2):291–5.
Josephson F, Andersson M, Flamholc L, Gisslén M, Hagberg L, Ormaasen V, et al. The relation between treatment outcome and efavirenz, atazanavir or lopinavir exposure in the NORTHIV trial of treatment-naïve HIV-1 infected patients. Eur J Clin Pharmacol. 2010;66(4):349–57.
Petersen K, Riddle M, Jones L, Furtek K, Christensen A, Tasker S, et al. Use of bilirubin as a marker of adherence to atazanavir-based antiretroviral therapy. AIDS. 2005;19(15):1700.
Karlström O, Josephson F, Sönnerborg A. Early virologic rebound in a pilot trial of ritonavir-boosted atazanavir as maintenance monotherapy. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2007;44(4):417.
Morello JE, Cuenca L, Vispo E. Use of serum bilirubin levels as surrogate marker of early virological response to atazanavir-based antiretroviral therapy. AIDS research and human retroviruses. 2011;27(00):2–5. doi:10.1089/aid.2011.0019.
Edén A, Andersson LM, Andersson Ö, Flamholc L, Josephson F, Nilsson S, et al. Differential effects of efavirenz, lopinavir/r, and atazanavir/r on the initial viral decay rate in treatment naïve HIV-1–infected patients. AIDS Research and Human Retroviruses. 2010;26(5):533–40.
Josephson F, Albert J, Flamholc L, Gisslén M, Karlström O, Lindgren SR, et al. Antiretroviral treatment of HIV infection: Swedish recommendations 2007. Scand J Infect Dis. 2007;39(6–7):486–507.
Gisslén M, Ahlqvist-Rastad J, Albert J, Blaxhult A, Hamberg AK, Lindbäck S, et al. Antiretroviral treatment of HIV infection: Swedish recommendations 2005. Scand J Infect Dis. 2006;38(2):86–103.
Beal SL SL, Boeckmann AJ (eds) Icon Development Solutions,, MD U, editors. NONMEM users guides. Ellicot City,1986–2006.
Jonsson EN, Karlsson MO. Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1999;58(1):51–64.
Wilkins JJ. NONMEMory: a run management tool for NONMEM. Comput Methods Programs Biomed. 2005;78(3):259–67.
Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79(3):241–57.
Dickinson L, Boffito M, Back D, Waters L, Else L, Davies G, et al. Population pharmacokinetics of ritonavir-boosted atazanavir in HIV-infected patients and healthy volunteers. Journal of Antimicrobial Chemotherapy. 2009;63(6):1233–43.
Anderson B, Holford N. Mechanism-based concepts of size and maturity in pharmacokinetics. Pharmacol Toxicol. 2008;48(1):303.
Jusko WJ, Ko HC. Physiologic indirect response models characterize diverse types of pharmacodynamic effects. Clin Pharmacol Ther. 1994;56(4):406–19.
Berk PD, Howe RB, Bloomer JR, Berlin NI. Studies of bilirubin kinetics in normal adults. J Clin Investig. 1969;48(11):2176.
Verotta D, Sheiner L. A general conceptual model for non-steady state pharmacokinetic/pharmacodynamic data. J Pharmacokinet Pharmacodyn. 1995;23(1):1–4.
Krzyzanski W, Jusko WJ. Indirect pharmacodynamic models for responses with multicompartmental distribution or polyexponential disposition. J Pharmacokinet Pharmacodyn. 2001;28(1):57–78.
Pocock S, Ashby D, Shaper A, Walker M, Broughton P. Diurnal variations in serum biochemical and haematological measurements. J Clin Pathol. 1989;42(2):172.
Manolio T, Burke G, Savage P, Jacobs Jr D, Sidney S, Wagenknecht L, et al. Sex- and race-related differences in liver-associated serum chemistry tests in young adults in the CARDIA study. Clin Chem. 1992;38(9):1853.
White Jr G, Nelson J, Pedersen D, Ash K. Fasting and gender (and altitude?) influence reference intervals for serum bilirubin in healthy adults. Clin Chem. 1981;27(6):1140.
Acknowledgements
The NORTHIV study was supported by grants from the Sahlgrenska Academy at the University of Gothenburg (ALFGBG-11067), the Research Foundation of Swedish Physicians Against AIDS, The Health & Medical Care Committee of the Region Västra Götaland (VGFOUREG 2591), the Swedish Research Council (2007-7092) and the Stockholm County Council ALF-grant (581513). We thank Dr. Filip Josephson for valuable discussions concerning bilirubin kinetics and Gilbert’s syndrome.
Disclosure
Daniel Röshammar is a current employee of AstraZeneca. However, this work is not sponsored by AstraZeneca or any other pharmaceutical company.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rekić, D., Clewe, O., Röshammar, D. et al. Bilirubin—A Potential Marker of Drug Exposure in Atazanavir-Based Antiretroviral Therapy. AAPS J 13, 598–605 (2011). https://doi.org/10.1208/s12248-011-9299-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-011-9299-0