Abstract
Adults with ambulatory hypertension or white coat hypertension (WCH) display abnormal cardiovascular rhythms. We studied cardiovascular rhythms by Fourier analysis of 24-h ambulatory blood pressure (BP) measurement profiles in 129 hypertensive children, 54 children with WCH, and 146 age-, height-, and gender-matched healthy subjects. The day/night mean arterial pressure ratio was lower in hypertensive and patients with WCH compared with controls (1.13 versus 1.16 versus 1.21, respectively; p < 0.0001). Eighty-five percent of controls were dippers compared with 74% of WCH (n.s.) and 64% of patients with ambulatory hypertension (p < 0.0001). The prevalence of 24-h rhythms was similar among the groups, but prevalence of 12-h BP rhythms was increased in hypertensive (67%) and WCH (72%) compared with controls (51%, p < 0.0001). The amplitudes of the 24-, 8-, and 6-h BP rhythms were reduced in hypertensive and WCH compared with controls (p < 0.05). Hypertensive and patients with WCH displayed delayed 24-, 12-, 8-, 6-h acrophases in comparison with controls (p < 0.05). In conclusion, hypertensive children exhibit abnormal cardiovascular rhythmicity compared with controls, especially a higher prevalence of nondipping compared with normotensive children. Abnormalities in patients with WCH are intermediate between healthy children and patients with ambulatory hypertension.
Similar content being viewed by others
Main
Similar to other functions of living organisms such as heart rate (HR), blood pressure (BP) also displays regular endogenous rhythmicity. Abnormalities of BP rhythms as assessed by analysis of ambulatory BP studies have been related to an increased risk of hypertensive target organ damage in adults (1–3).
Although primary hypertension (PH) has its origins very early in childhood and adolescence, there are only scanty reports on BP rhythmicity in hypertensive children. There are no data comparing BP rhythmicity of healthy, normotensive children with hypertensive children and children with white coat hypertension (WCH). Moreover, it is still unclear whether WCH is only a benign form of enhanced reactivity not related to pathologic BP regulation or a true pathologic state related to disturbed BP rhythmicity, representing a prehypertension condition. Finally, because hypertension in adolescents is mainly linked with obesity, it is important to assess whether obesity has any independent impact on BP rhythmicity (4,5).
We hypothesized that, similar to adults, children and adolescents with sustained ambulatory hypertension and WCH also have disturbed BP rhythms. To evaluate our hypothesis, we analyzed BP rhythmicity retrospectively in cohorts of untreated children with PH and WCH and compared the results with normative data obtained from healthy normotensive children (6).
METHODS
Patients.
We included 183 children and adolescents referred to two tertiary centers (Children's Memorial Health Institute, Warsaw, Poland and Children's Hospital of Eastern Ontario, Ottawa, Canada) for further evaluation of hypertension based on an elevated office BP confirmed on at least three visits. All patients underwent the same diagnostic work up at both institutions. PH was diagnosed after a thorough clinical and laboratory diagnostic work up, according to recently published recommendations (7). Office BP values, measured with an oscillometric device (Dinamap 1846 SX; Criticon Inc., Tampa, FL) after at least 5 min of rest in a quiet room, were compared with normative values obtained from the Updated Fourth Task Force Report (7).
All the patients underwent ambulatory BP monitoring (ABPM). Those with normal ABPM (24 h, daytime and nighttime mean arterial BP below the 95th percentile) but elevated office BP (either systolic or diastolic BP >95th percentile) were classified as having WCH, and those with abnormal ABPM and elevated office BP were classified as having ambulatory hypertension. Patients with WCH and ambulatory hypertension were analyzed as separate groups. To define the effect of obesity, both hypertensive and WCH groups were further subdivided into obese and nonobese groups on the basis of a BMI below and above the 95th percentile derived from the US reference data for Canadian children and polish normative data for children evaluated in Warsaw (8,9).
The control group consisted of 146 age-, height-, and gender-matched healthy subjects studied in Germany (14.6 ± 2 y) who were described elsewhere (6,10) and representing the whole spectrum of BP values of the healthy childhood population (i.e. also including children with BP values below the fifth or above the 95th centile for height on ABPM).The study adhered to the principles of the Declaration of Helsinki and was approved by local Ethical Committees in all participating centers. Informed consent has been obtained from all control subjects, hypertensive patients, and their parents.
ABPM measurements.
All ABPM measurements were assessed oscillometrically using SpaceLabs Monitor 90207, and the most appropriate cuff was applied on the nondominant arm. Readings were taken every 20 min during day time and every 30 min at night. Recordings lasting at least 20 h with at least 80% of readings were considered as valid and were included in the analysis. Absolute BP values obtained from ABPM were subsequently transformed into SD scores (SDS) based on normative ABPM values (11). Because during oscillometric measurements only mean arterial BP is measured (representing the BP value with the greatest oscillations) and systolic and diastolic BPs are mathematically derived with device-specific algorithms, we analyzed only mean arterial pressure (MAP).
BP nighttime dipping status was analyzed by calculating the ratio of MAP during the day (D) in relation to nighttime (N) MAP (MAP D/N). Similar to the study by Wuhl et al. (11), the MAP lowering during the night below 10% was considered as dipping. MAP nighttime dipping of <10% (MAP D/N < 1.1 and > 1.0) was considered as nondipping. Similarly, the HR nighttime dipping was described with the daytime/nighttime ratio (HR D/N). The night period was defined from 0000 h until 0600 h, and the daytime period was defined as 0800 h until 2000 h.
Rhythm analysis.
Rhythm analysis was performed according to the procedure described elsewhere (6). In short, the 24-, 12-, 8-, and 6-h BP and HR rhythms were analyzed using Fourier analysis. First, the 24-h circadian rhythm was checked using least squares analysis and was considered as present if a cosine function within 24 h was fitted with p < 0.05. Then, shorter ultradian rhythms were described in the same manner.
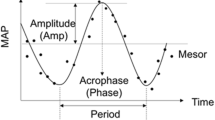
The following parameters were calculated for each significant rhythm: 1) MESOR, which is the median value between the lowest and highest values of the fitted cosine curve, 2) amplitude, which is the difference between MESOR and highest value, and 3) acrophase, which is the time from midnight to the highest value during the rhythm.
Statistical analysis.
Subject characteristics and results are presented as mean ± SD. BMI and BP values were expressed both as absolute values and as SDS corrected for age and gender, because the analyzed groups included subjects of different age and gender. Homogeneity of variance was checked with the Levene test. Variables with a normal distribution were compared by the t test for independent variables. Values with nonnormal distribution were compared by the Mann-Whitney U test. Three groups of patients and controls were compared using ANOVA test. Correlation analysis was done with Pearson test for variables with parametric distribution and Spearman test for variables with nonparametric distribution. A p value < 0.05 was regarded as significant. A p value ranging between 0.05 and 0.1 was regarded as a statistical tendency.
RESULTS
One hundred twenty-nine children were diagnosed with ambulatory PH (mean age, 14.7 ± 2.6 y), and 54 children had WCH (mean age, 14.5 ± 3.0 y). The study and control groups did not differ regarding age and height, but patients with PH and WCH had greater BMI than controls (Table 1). Both patients with PH and WCH had a higher average BP and lower day/night MAP ratio compared with controls. Although the mean 24-h HR was the same in the three groups, patients with PH had higher HR during night in comparison with WCH and healthy children (Table 1).
Eighty-five percent of healthy children were dippers compared with 64% of PH (p < 0.0001) and 74% of WCH (n.s.). On the contrary, 54 (37%) of healthy controls displayed lowering of MAP by >20% that was significantly more prevalent in comparison with only 5 (4%) of patients with PH (p < 0.0001) and 9 (17%) of patients with WCH (p = 0.02). Such a reaction was significantly more often detected in patients with WCH in comparison with patients with PH (p = 0.007). Accordingly, the nighttime decrease of HR was significantly more pronounced in WCH in comparison with patients with PH (p = 0.03; Table 1).
The prevalence of circadian 24-h MAP rhythms was similar among the groups; they were present in >80% of patients and controls. However, the prevalence of the ultradian 12-h MAP rhythms was significantly increased in PH (67%) and WCH (72%) versus controls (51%, p < 0.0001). The prevalence of the 8-h MAP rhythms was similar in all groups and was present in ∼30% of patients and controls (Fig. 1). The prevalence of 24-h HR rhythm was the same in all groups, but prevalence of the 12-h HR rhythm was lower in patients with PH (48%) in comparison with controls (61%, p = 0.03) and patients with WCH (64%, p = 0.04).
The amplitudes of the 24-, 8-, and 6- MAP rhythms were reduced in both patients with PH and WCH compared with controls (p < 0.05). The same was observed for 12-, 8-, and 6-h HR rhythms (Fig. 2). Patients with PH and WCH displayed significantly prolonged acrophases of MAP rhythms compared with controls; patients with PH had the longest acrophase of the 24-h MAP rhythm in comparison with patients with WCH and controls. The same pattern was found for HR acrophases (Fig. 2).
Amplitudes (A, MAP; B, HR) and acrophases (C, MAP; D, HR) of the 24-, 12-, 8- and 6-h rhythms in children with PH (n = 129, ▪), children with WCH (n = 54,  ), and healthy normotensive children (n = 146, □). Box plots indicate 25th, 50th (median), and 75th percentiles, whisker 10th and 90th percentiles of healthy children. * indicates significant (p < 0.05) differences between children with PH or WCH and healthy normotensive children. ** indicates significant (p < 0.05) differences between children with PH and children with WCH.
), and healthy normotensive children (n = 146, □). Box plots indicate 25th, 50th (median), and 75th percentiles, whisker 10th and 90th percentiles of healthy children. * indicates significant (p < 0.05) differences between children with PH or WCH and healthy normotensive children. ** indicates significant (p < 0.05) differences between children with PH and children with WCH.
Obese hypertensive patients (n = 54) displayed similar 24 h, daytime and nighttime MAP values as lean hypertensive patients (24-h MAP SDS: n = 75, 2.1 ± 1.8 versus 1.8 ± 1.4; n.s.). Nevertheless, the 24-h MAP amplitudes were lower in nonobese patients with PH in comparison with obese patients with PH (8.4 ± 2.9 versus 10.0 ± 3.4 mm Hg; p = 0.02). Although the 24-h MAP acrophases were similarly prolonged in both obese and nonobese hypertensive children in comparison with controls, the 12-h MAP acrophase was delayed (9.1 ± 2.5 versus 7.9 ± 2.7; p = 0.01), whereas the 8-h MAP acrophase was premature (3.2 ± 1.9 versus 4.5 ± 2.1; p = 0.04) in lean patients with PH in comparison with obese patients with PH (Fig. 3).
Acrophases of the 24-, 12-, 8- and 6-h MAP rhythms in obese (n = 54,  ) and lean (n = 75, ▪) children with PH, compared with normotensive obese children (n = 15, □). Box plots indicate 25th, 50th (median) and 75th percentiles, whisker 10th and 90th percentiles of healthy children. ** indicates significant (p < 0.05) differences between obese and lean children with PH.
) and lean (n = 75, ▪) children with PH, compared with normotensive obese children (n = 15, □). Box plots indicate 25th, 50th (median) and 75th percentiles, whisker 10th and 90th percentiles of healthy children. ** indicates significant (p < 0.05) differences between obese and lean children with PH.
To evaluate further potential effects of obesity on BP and HR rhythmicity, obese children were analyzed separately. Obese patients with PH (n = 54) had significantly higher HR at night and lower day/night ratio of HR and BP compared with obese normotensive children (n = 15); however, the amplitudes and the acrophases were similar (Fig. 3).
Although there were some correlations between BMI-SDS and amplitudes or acrophases in hypertensive patients, the significance and r coefficients were weak (24-h MAP amplitude: r2 = 0.034, p = 0.05; 8-h MAP acrophase: r2 = 0.12, p = 0.02).
DISCUSSION
The main finding of our study is that children with ambulatory hypertension and WCH display significantly altered circadian and ultradian BP and HR rhythmicity patterns in comparison with healthy, normotensive children. Moreover, patients with WCH manifest an intermediate pattern of altered BP/HR rhythms in comparison with both healthy children and children with ambulatory hypertension. Finally, we did not find any strong and independent effect of obesity on BP rhythmicity.
The circadian rhythm of cardiovascular functions is generated by a central pacemaker probably located in the suprachiasmatic nuclei and is transmitted through efferent sympathetic nerves (12,13). The shorter, ultradian rhythms are more dependent on sympathetic activity caused by external and behavioral stimuli (14). Although it is generally believed that alterations in biologic rhythms indicate a disturbed central regulatory function, abnormal baroreceptor function could also be involved. Indirect evidence suggests that in hypertensive children, the function of central sympathetic centers is disturbed and leads to increased and prolonged central sympathetic drive or inability to increase parasympathetic activity during night (15,16).
BP dipping is the most commonly used marker of BP rhythmicity, and a blunted nocturnal dip is strongly independently related to target organ damage and greater cardiovascular risk in hypertensive adults (1–3). Most studies examining the dipping phenomenon in pediatric hypertension concentrated on secondary hypertension. Disturbed dipping was found in children with renal scarring and children with chronic kidney disease in whom prevalence of nondipping was 35–21% in comparison with 10–13% in healthy controls (17–19). Some authors treat a reduced nocturnal BP dip and sustained nighttime hypertension as specific markers for secondary hypertension when compared with PH (20). Moreover, nondipping in normotensive (by casual BP measurements) children with type 1 diabetes predicted microalbuminuria (21,22). There are only scanty reports on dipping status in children with PH. Diaz and Garin (23) reported that 40% of children with PH were nondippers. Because obesity is a frequent phenotype of PH in childhood, it is important to define the relation between obesity and the nocturnal fall of BP. Nocturnal dipping was found less marked in obese than in lean children and a weak, but a significant negative correlation between dipping and BMI-SDS was observed (24). Marcovecchio et al. (25) found that 24 of 56 obese children were nondippers, and ABPM parameters correlated with markers of insulin resistance. In a recent study by Gilardini et al. (26), nondipping patterns were found in 11% of obese children, and the loss of nocturnal BP fall correlated with sympathoadrenergic drive as indicated by catecholamine excretion and insulin resistance. In addition, Shatat et al. (27) found that a significant number of obese children displayed nondipping phenomenon, and BP values were inversely correlated with adiponectin concentrations; however, they did not report any relation with dipping.
Given some uncertainty and heterogeneity of the definition of dipping/nondipping in children in previous studies, we used the MAP for the definition of dipping/nondipping, which is consistent with two recent pediatric studies and normative values for cardiovascular rhythms in childhood (6,19). We found that 15% of healthy children, 26% of WCH, and 36% of patients with ambulatory hypertension were nondippers. In addition, there was a significant gradual decrease of the day/night MAP ratio from normotension through WCH to the ambulatory hypertensive group. Moreover, 37% of healthy children displayed an overdipping pattern, i.e. nocturnal lowering of BP by >20%, which was significantly more frequent than in WCH (17%) and hypertensive (4%) patients. This finding suggests both prolonged and greater basal sympathetic activity in WCH and hypertensive patients.
Because both patients with WCH and ambulatory hypertension had significantly greater BMI than controls, it is difficult to dissect the effects of obesity on BP dipping. Moreover, when obese normotensive children were compared with BMI matched (obese) patients with ambulatory hypertension, the nighttime drop of BP and HR was significantly lower in hypertensives. This suggests that the nondipping phenomenon in hypertensives is not primarily related to obesity but rather to a primary disturbance of the BP rhythm. The same concerns the lack of differences in BP values between lean and obese hypertensives. However, it must be underlined that we did not analyze effects of visceral obesity and insulin resistance on cardiovascular rhythmicity.
It was recently found that in obese adolescents, BMI correlates with BP and that insulin resistance correlates with nocturnal but not with awake BP (28). However, in our study obese, patients with ambulatory hypertension had slightly not significantly lower BP values than lean hypertensives. This finding may support the view that obesity-related hypertension is pathogenetically different from PH in lean children. Similarly, when obese normotensive children were compared with the subgroup of obese, hypertensive patients, it was found that obese hypertensives displayed higher nighttime HR and a weaker nighttime decrease of BP (nondipping). These findings suggest that hypertension is related to disturbances of the central sympathetic oscillator independently of BMI.
The increasing prevalence of nondipping from normotensive through patients with WCH to ambulatory hypertension indicates that WCH in hemodynamic terms is an intermediate form of altered BP control. On the other hand, both the lower prevalence of nondipping and the significantly higher prevalence of overdipping in healthy children indirectly suggest that children with WCH and ambulatory hypertension have an increased sympathetic drive in comparison with healthy children.
We did not find any significant differences in the prevalence of circadian 24-h and short 8-h BP rhythms between groups (6). In contrast, the 12-h rhythm was significantly more prevalent in both patients with WCH and ambulatory hypertension than in healthy children. Because the ultradian BP rhythmicity is related to sympathetic drive, the finding of a more prevalent 12-h rhythm in WCH and hypertensive patients and the same circadian and 8-h rhythmicity may indicate similar reactivity with prolonged duration of increased sympathetic activity during wakefulness in hypertensive children. It would also support the concept that sympathetic system plays a major role in the pressor effects of environmental stimuli operating during daytime.
The amplitudes of all rhythms were lower, and acrophases were prolonged in patients with WCH and ambulatory hypertension compared with healthy children. It was related to greater MESOR values what is virtually the same as 24-h MAP. This blunting of amplitudes and delayed acrophases of the BP rhythms are similar in extent to children with chronic kidney disease as previously reported (19). Again, because ultradian rhythmicity depends in large part on sympathetic drive, one can assume that prolonged acrophases in hypertensive patients may reflect prolonged effects of an increased sympathetic activity (26,29,30).Our study has some limitations such as the lack of data on metabolic abnormalities and target organ damage, precluding the analysis of a potential relationship between altered BP rhythmicity and early, subclinical injury in childhood hypertension.
There are some potential perspectives provided by our data: first, it will be important to evaluate whether altered cardiovascular rhythmicity correlates with target organ damage in hypertensive children. Second, there is a need to define potential effects of different therapeutic interventions on cardiovascular rhythmicity pattern, including effects of behavioral and physical exercise interventions and effects of different pharmacological agents. Third, although we analyzed relations between BMI and BP/HR rhythmicity, it remains to be elucidated whether there are relationships with fat tissue distribution, adiposity, and visceral obesity. It is also unknown whether there is a link between metabolic abnormalities (including insulin resistance) and cardiovascular rhythmicity in PH, and whether normalization of metabolic abnormalities would normalize altered cardiovascular rhythmicity.
Abbreviations
- ABPM:
-
Ambulatory blood pressure monitoring
- BP:
-
Blood pressure
- HR:
-
Heart rate
- MAP:
-
Mean arterial pressure
- PH:
-
Primary hypertension
- WCH:
-
White coat hypertension
References
Cuspidi C, Meani S, Valerio C, Fusi V, Zanchetti A 2006 Nocturnal non-dipping pattern in untreated hypertensives at different cardiovascular risk according to the 2003 ESH/ESC guidelines. Blood Press 15: 37–44
Cuspidi C, Meani S, Salerno M, Valerio C, Fusi V, Severgnino B, Lonati L, Magrini F, Zanchetti A 2004 Cardiovascular target organ damage in essential hypertension with or without reproducible nocturnal fall in blood pressure. J Hypertens 22: 273–280
Routledge FS, McFetridge-Durdle JA, Dean CR, Canadian Hypertension Society 2007 Night-time blood pressure patterns and target organ damage: a review. Can J Cardiol 23: 132–138
Robinson RF, Batisky DL, Hayes JR, Nahata MC, Mahon JP 2004 Body mass index in primary and secondary hypertension. Pediatr Nephrol 19: 1379–1384
Flynn JT, Alderman MH 2005 Characteristics of children with primary hypertension seen at a referral center. Pediatr Nephrol 20: 961–966
Hadtstein C, Wuhl E, Soergel M, Witte K, Schaefer F 2004 German Study Group for pediatric hypertension normative values for circadian and ultradian cardiovascular rhythms in childhood. Hypertension 43: 547–554
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents 2004 The fourth report on diagnosis, evaluation and treatment of high blood pressure in children and adolescents. Pediatrics 114: 555–576
Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL 2002 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11: 1–190
Palczewska I, Niedzwiedzka Z 2001 Somatic development indices in children and youth of Warsaw [in Polish]. Med Wieku Rozwoj 5: 18–118
Soergel M, Kirschstein M, Busch C, Danne T, Gellermann J, Holl R, Krull F, Reichert H, Reusz GS, Reicher W 1997 Oscillometric twenty-four-hour ambulatory blood pressure values in healthy children and adolescents: a multicenter trial in children. J Pediatr 130: 178–184
Wuhl E, Witte K, Soergel M, Mehls O, Schaefer F, German Working Group on Pediatric Hypertension 2002 Distribution of 24-h ambulatory blood pressure in children: normative reference values and role of body dimensions. J Hypertens 20: 1995–2007
Kalsbeek A, Perreau-Lenz S, Buijs RM 2006 A network of clock (autonomic) outputs. Chronobiol Int 23: 521–535
Diedrich A, Jordan J, Tank J, Shannon JR, Robertson RM, Luft FC, Robertson D, Biaggoni I 2003 The sympathetic nervous system in hypertension: assessment by blood pressure variability and ganglionic blockade. J Hypertens 21: 1677–1686
Narkiewicz K, Winnicki M, Schroeder K, Phillips BG, Kato M, Cwalina E, Sommers VK 2002 Relationship between muscle sympathetic nerve activity and diurnal blood pressure profile. Hypertension 39: 168–172
Sherwood A, Steffen PR, Blumenthal JA, Kuhn C, Hinderliter AL 2002 Nighttime blood pressure dipping: the role of autonomic nervous system. Am J Hypertens 15: 111–118
Kohara K, Nishida W, Maguchi M, Hiwada K 1995 Autonomic nervous function in non-dipper essential hypertensive subjects. Evaluation by power spectra analysis of heart rate variability. Hypertension 26: 808–814
Patzer L, Seeman T, Luck C, Wuhl E, Janda J, Misselwitz J 2003 Day and nighttime blood pressure elevation in children with higher grades of renal scarring. J Pediatr 142: 117–122
Lingens N, Freud M, Seeman T, Witte K, Lemmi B, Scharer K 1997 Circadian blood pressure changes in untreated children with kidney disease and conserved renal function. Acta Paediatr 86: 719–723
Wuhl E, Hadtstein C, Mehls O, Schaefer F, ESCAPE Trial Group 2005 Ultradian but not circadian blood pressure rhythms correlate with renal dysfunction in children with chronic renal failure. J Am Soc Nephrol 16: 746–754
Seeman T, Palyzova Dusek J, Janda J 2005 Reduced nocturnal blood pressure dip and sustained nighttime hypertension are specific markers of secondary hypertension. J Pediatr 147: 366–371
Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, Alvarez V, Battle D 2002 Increase in nocturnal blood pressure and progression to microalbuminuria in type I diabetes. N Engl J Med 347: 797–805
Dost A, Klinkart C, Kapellen T, Lemmer A, Naeke A, Grabert M, Kreuder J, Holl RW 2008 Arterial hypertension defined by ambulatory blood pressure profiles: contribution to microalbuminuria risk in a multicenter investigation in 2105 children and adolescents with type 1 diabetes. Diabetes Care 31: 720–725
Diaz LN, Garin EH 2007 Comparison of ambulatory blood pressure and task force criteria to identify pediatric hypertension. Pediatr Nephrol 22: 554–558
Framme J, Dongevalt F, Merilol S, Osika W, Wahrberg P, Friberg P 2006 24-h systolic blood pressure and heart rate reactivity in lean and obese adolescents. Clin Physiol Funct Imaging 26: 235–236
Marcovecchio ML, Patricelli L, Zito M, Cepanne R, Ciampani M, Chiarelli F, Mohn A 2006 Ambulatory blood pressure monitoring in obese children: role of insulin resistance. J Hypertens 24: 2431–2436
Gilardini L, Parati G, Sartorio A, Mazzilli G, Pontiggia B, Inviti C 2008 Sympathoadrenergic and metabolic factors are involved in ambulatory blood pressure rise in childhood obesity. J Hum Hypertens 22: 75–82
Shatat IF, Freeman KD, Vuguin PM, Dimartino-Nardi JR, Flynn JT 2009 Relationship between adiponectin and ambulatory blood pressure in obese adolescents. Pediatr Res 65: 691–695
Lurbe E, Torro I, Aquilar F, Alvarez J, Alcon J, Pascual JM, Redon J 2008 Added impact of obesity and insulin resistance on blood pressure elevation in children and adolescents. Hypertension 51: 635–641
Mark AL 1996 The sympathetic nervous system in hypertension: a potential long-term regulator of arterial pressure. J Hypertens Suppl 14: S159–S165
Somers VK, Dyken ME, Mark AL, Abboud FM 1993 Sympathetic nerve activity during sleep in normal subjects. N Engl J Med 328: 303–307
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported in part by the AstraZeneca Scholarship of the Swiss Society of Hypertension [G.D.S.].
Rights and permissions
About this article
Cite this article
Litwin, M., Simonetti, G., Niemirska, A. et al. Altered Cardiovascular Rhythmicity in Children With White Coat and Ambulatory Hypertension. Pediatr Res 67, 419–423 (2010). https://doi.org/10.1203/PDR.0b013e3181d00b5b
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e3181d00b5b
This article is cited by
-
Obesity, metabolic syndrome, and primary hypertension
Pediatric Nephrology (2021)
-
Why should we screen for arterial hypertension in children and adolescents?
Pediatric Nephrology (2018)