Abstract
We hypothesized that resuscitation with 100% O2 compared with 21% O2 is detrimental to pulmonary tissue. The pulmonary injury was assessed by matrix metalloproteinase (MMP) activity, oxidative stress, IL-8, and histology 2.5 h after resuscitation from a hypoxic state. In pulmonary tissue extracts, MMP activity was analyzed by broad matrix–degrading capacity (total MMP) and zymography. MMP-2 mRNA expression was evaluated by quantitative real-time PCR. Total endogenous antioxidant capacity was measured by the oxygen radical absorbance capacity (ORAC) assay, and IL-8 was analyzed by ELISA technique. In bronchoalveolar lavage (BAL) fluid, MMPs were analyzed by zymography. In pulmonary tissue, pro- and active MMP-2 levels were increased in piglets that were resuscitated with 100% O2 compared with 21% O2. Pro–MMP-9, total MMP activity, and MMP-2 mRNA levels were significantly increased in resuscitated piglets compared with baseline. Net gelatinolytic activity increased in submucosa and blood vessels after 100% O2 and only in the blood vessels after 21% O2. Compared with baseline, ORAC values were considerably lowered in the resuscitated piglets and significantly reduced in the 100% O2 versus 21% O2 group. In BAL fluid, both pro–MMP-9 and pro–MMP-2 increased 2-fold in the 100% O2 group compared with 21% O2. Moreover, IL-8 concentration increased significantly in piglets that were resuscitated with 100% O2 compared with 21% O2, suggesting a marked proinflammatory response in the pulmonary tissue. Altogether, these data strongly suggest that caution must be taken when applying pure O2 to the newborn infant.
Similar content being viewed by others
Main
Traditionally, asphyxiated newborn infants have been resuscitated with 100% O2. This recommendation is based mainly on precedent rather than sound evidence. Hyperoxia leads to generation of O2 free radicals, which have a role in reperfusion injury after asphyxia (1). Naturally, the lung is exposed directly to the highest partial pressure of inspired O2, and pulmonary damage, as a result of O2 exposure, is a serious clinical complication in infants who require high levels of O2 as treatment (2). It is important not only to provide adequate O2 consumption in organs but also to prevent further tissue damage during resuscitation caused by reoxygenation injury or O2 toxicity. This might be achieved by lowering the O2 concentration during resuscitation. There is therefore an ongoing debate whether to use 21% or 100% O2 in neonatal resuscitation (3).
Matrix metalloproteinases (MMPs) are involved in the pathogenesis of tissue inflammation and wound healing in lung injury (4,5). The role of oxidative stress and its toxic effects on lipids, as well as on disruption of extracellular matrix through up-regulation of MMPs, is well established (6,7). Reduced antioxidant capacity and low concentrations of tissue inhibitors of MMPs (TIMPs) seem to predispose the preterm infant to tissue damage (8). Over expression of MMPs has been implicated in the pathogenesis of pulmonary diseases in premature infants (6). One of the mechanisms of action of MMPs in the pathogenesis of tissue damage relies on the breakdown of constituents of the extracellular matrix. MMP-2 (72-kDa gelatinase or gelatinase A) is secreted mainly by noninflammatory cells as fibroblasts and endothelial and epithelial cells, whereas MMP-9 (92-kDa gelatinase or gelatinase B) is secreted by inflammatory cells such as neutrophils and monocyte-macrophages (9). The expression of MMPs is regulated by several factors, such as cytokines, growth factors, and extracellular matrix components (10). Several studies have investigated the roles of these proteinases in acute lung injury. However, their precise contribution in the development of acute hyperoxic lung injury, including the relationship among MMPs, severity of disease, and pathologic changes, is still unclear (11). MMPs play a role in the increased pulmonary permeability and inflammation after oxidative stress. MMP-2 and MMP-9 have a capacity to degrade gelatin, elastin, fibronectin, and type IV collagen, all of which are major structural components of the basal membrane (12); in addition, they proteolytically activate cytokines such as IL-8 (13). Hyperoxia is also known to stimulate the alveolar macrophages to produce chemokines such as IL-8 into the alveolar space, which (11) up-regulates MMP-2 and MMP-9 production (14). Overproduction of proinflammatory cytokines has been suggested to be a major factor associated with pulmonary damage. In vivo, IL-8 level is abundantly expressed in the lungs of animal models during O2 injury (15) and in human premature infants who develop bronchopulmonary dysplasia (16,17).
Most previous studies have been carried out in cell lines or animals with few similarities with humans. Therefore, in the present study we investigated pulmonary changes in piglet, which resembles the human lung anatomically and immunologically (18,19). The main aim of this study was to explore whether resuscitation with 100% O2 compared with ambient air increases the acute pulmonary damage after global hypoxia in a piglet model. Birth asphyxia is often associated with hypercapnia, and arterial carbon dioxide tension (Paco2) influences pulmonary circulation (20). Consequently, we also investigated effects of different Paco2 levels during resuscitation.
METHODS
The Norwegian Council for Animal Research approved the experimental protocol. The animals were cared for and handled in accordance with the European Guidelines for Use of Experimental Animals.
Surgical preparation and anesthesia.
Sixty-nine newborn anesthetized and tracheotomized Landrace pigs (12–36 h old) were included in the study. The piglets were anesthetized, ventilated, and surgically prepared as previously described (21).
Experimental protocol.
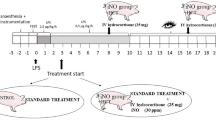
The animals were stabilized for 1 h after the initial procedures. Hypoxemia was achieved by ventilation with a gas mixture of 8% O2 in N2 until either mean arterial blood pressure decreased to 15 mm Hg or base excess (BE) reached −20 mM. CO2 was added during hypoxemia at a Paco2 8.0–9.5 kPa, aiming to imitate birth asphyxia. Before start of resuscitation, the piglets were block-randomized into six different groups. Resuscitation was performed for 30 min with either 21% O2 (group A) or 100% O2 (group B). Nine piglets, referred to as baseline pigs, were controls that went through surgery and 1 h of stabilization. Each main group A and B was further divided into three subgroups that were ventilated with low (A1 and B1), normal (A2 and B2), or high (A3 and B3) Pco2 level during resuscitation. Subgroup 1 (A1 n = 10/B1 n = 10) were hyperventilated, (Paco2 2.0–3.5 kPa). Subgroups 2 and 3 were ventilated in a normal ventilatory modus. In group 2 (A2 n = 10/B2 n = 10), Paco2 was 4.5–6.0 kPa. Subgroup 3 (A3 n = 10/B3 n = 10) had CO2 added to reflect hypoventilation (Paco2 8.0–9.5 kPa). For hyperventilating the piglets (group 1), peak inspiratory pressure and ventilatory rate were elevated and adjusted after evaluating end-tidal Pco2 and blood gases. Furthermore, the piglets were reoxygenated for 150 min by 21% O2 and normal Paco2 (4.5–6.0 kPa), which was the same for all of the groups. Bronchoalveolar (BAL) fluid was collected at baseline and at the end of the experiment by instillation of 1 mL/kg sterile saline into the endotracheal tube via a 5-F gauge feeding catheter that had been advanced through the endotracheal tube. The piglet was manually bagged three times, and the fluid was immediately re-aspirated and centrifuged at 3000 × g for 5 min at room temperature, and the supernatant was aspirated and stored at −70°C for further analysis. At the end of the experiment, the piglets were given an i.v. overdose of 150 mg/kg pentobarbital. The left lung was immediately frozen on liquid nitrogen and stored at −70°C, and the right lung was stored in formalin.
Preparation of pulmonary tissue extracts.
For zymography and total MMP activity, pulmonary tissue from the left lower lung lobe was pulverized under liquid nitrogen, and proteins were extracted using ice-cold lysis buffer [Tris-HC (pH 7.5) that contained 1% NP-40 and a protease inhibitor cocktail without EDTA] at a ratio of 500 μL/50 mg wet weight tissue. Extracts of lung tissue were incubated on ice for 15 min and then centrifuged at 12,000 × g for 15 min at 4°C. The supernatants were retained, and protein concentration of the samples was measured by BCA method (Pierce, Cheshire, UK). For the oxygen radical absorbance capacity (ORAC) assay, pulmonary tissue was pulverized under liquid nitrogen, and 50 mg of frozen material was added to 1.0 mL of ice-cold 75 mM K2HPO4/NaH2PO4 phosphate buffer (pH 7.0). The tissue samples were homogenized using a Polytron (PT 1200; Kinematica AG, Lucerne, Switzerland) at speed 1 for 30 s under nitrogen flush and centrifuged at 16,000 × g for 5 min at 4°C (Biofuge A; Hereaeus-Christ, Hanau, Germany). The pellet was resuspended in homogenization buffer and centrifuged as described above. The two supernatants were combined in a volumetric flask, filled up to 2.0 mL of total volume, and used for the ORAC assay.
Total MMP activity.
Total MMP activity in tissue extracts was measured using a fluorogenic peptide substrate (cat no. ES001; R&D Systems, Minneapolis, MN) according to the protocol recommended by the manufacturer. The substrate can be cleaved by MMP-1, MMP-2, MMP-7, MMP-8, MMP-9, MMP-12, and MMP-13. The term “total MMP activity” refers to the total activity of these enzymes, and the activity is expressed by relative fluorogenic unit. Because of limited amount of tissue, data from nine animals were not obtained (three at baseline, three in groups A and B; baseline, n = 5; A, n = 27; and B, n = 27).
Gelatin zymography.
Equal amounts (10 μg) of lung tissue and BAL fluid protein extracts were loaded onto the electrophoretic gel and assayed for gelatinase activity using 10% SDS-polyacrylamide gel (containing 0.1% gelatin), with minor modifications according to the methods described elsewhere (22). Human MMP-2 and MMP-9 standards (CC073; Chemicon, Temecula, CA) were used. The gels were stained with Coomassie blue R-250 and subsequently destained before being scanned in an imager (Kodak Image Station 440CF, Rochester, NY). The images were analyzed using software from Total Lab v2.01 (Newcastle upon Tyne, UK). The results were calculated by densitometry, normalized to a sample used as an internal standard on every zymography run. When the gelatin gel was incubated with 200 μM EDTA, no lysis zones were detected, demonstrating that the metal-dependent lysis zones were most likely the result of gelatinase activity (23). Because of limited amount of tissue, data from five animals were not obtained (one at baseline, two in group A, and two in group B; baseline: n = 8; A, n = 28; B, n = 28). BAL fluid analyses were performed on piglets in group A2 and B2. Because of the limited BAL fluid, data from one animal were not obtained in group A (group A, n = 9; group B, n = 10).
In situ zymography.
In situ zymography was performed to localize net gelatinolytic activity in lung sections with a few modifications with respect to a method previously described in brain tissue (24). Fresh frozen lung sections (40 μm thick) were generated using a cryostat (Leica CM3050S, Nussloch, Germany). Nonfixed lung sections were incubated for 2 h at 37°C in a humid dark chamber in a reaction buffer that contained 0.5 M Tris-HCl, 1.5 M NaCl, 50 mM CaCl2, 2 mM sodium azide (pH 7.6), and 80 μg/mL FITC-labeled DQ-gelatin (EnzCheck collagenase kit; Molecular Probes, Eugene, OR) that is intramolecularly quenched. After the incubation, tissue was fixed in 4% paraformaldehyde (Acros, Elancourt, France), incubated for 5 min with 0.5 μg/mL Hoechst 33258 (Molecular Probes, Leiden, the Netherlands), and mounted in fluorescent mounting medium (Dako, Carpinteria, CA). Some sections were incubated with 1 mM phenanthrolene (Molecular Probes), a broad-spectrum MMP inhibitor. Samples were observed with a fluorescent microscope (E800; Nikon, Champigny-sur-Marne, France) equipped with FITC and DAPI filters, and images were analyzed using a DXM 1200 camera and the Lucia software (Nikon). Gelatin-FITC cleavage by tissue gelatinases releases quenched fluorescence representative of net proteolytic activity. Sections incubated without DQ-gelatin were not fluorescent. We used five piglets per experimental group and analyzed three slices per animal.
Real-time quantitative reverse transcriptase–PCR.
Real-time quantitative reverse transcriptase–PCR (RT-PCR) was done as previously described (21). Total RNA was extracted from the tissue samples using Trizol (Invitrogen, San Diego, CA) and treated with DNase (cat no. M6101; Promega, Madison, WI). Reverse transcription was performed (cat no. N808-0234; Applied Biosystems, Foster City, CA) with 125 ng of RNA per 50 μL of RT reaction. Quantification of mRNA was performed using the ABI Prism 7700 (Applied Biosystems). Sequence-specific PCR primers and TaqMan Probe for porcine MMP-2 was used (forward primer: 5′-GTG GTG CGT GTG AAG TAT GG-3′; reverse primer: 5′-GCC ATC CTT GTC GAA GTT GT-3′; TaqMan probe: FAM 5′)-AGC TGT TAT ACT CCT TGC CGT T-TAMRA-3′). The housekeeping gene 18S (Applied Biosystems) was included as an endogenous normalization control to adjust for unequal amounts of RNA. Standard curves were run on the same plate, and the relative standard curve method was used to calculate the relative gene expression. Real-time quantitative RT-PCR was performed on samples from groups A2 and B2. Because of the limited amount of tissue, data from two animals were not obtained (one at baseline and one in group B; baseline, n = 8; A, n = 10; B, n = 9).
ORAC assay.
The antioxidant capacity of the pulmonary tissue extracts was determined by the ORAC assay (25) and modified for measurements on the Wallac 1420 VICTOR2 96-well microplate reader (Perkin-Elmer Life and Analytical Sciences, Wallac Oy, Turku, Finland) (21,26). In this assay, a fluorescent protein β-phycoerythrin was used as an indicator, 2,2′-azobis (2-amidinopropane) dihydrochloride as the source for the peroxyl radicals, and Trolox (a water-soluble vitamin E analogue) as a control standard. The extent of protection against the peroxyl radicals by the antioxidants in the sample was measured every 2 min until the fluorescence was <5% of the initial reading. The fluorescence measurements were expressed relative to the initial reading. Results were calculated by using the area differences under the β-phycoerythrin decay curves between the blank and the sample. The final results (ORAC values) were calculated by linear regression of areas versus sample concentration and expressed as micromoles of Trolox equivalents (TE) per gram of pulmonary tissue (μmol TE/g). Because of the limited amount of tissue, data from two animals were not obtained (one at baseline and one in group B; baseline, n = 8; A, n = 10; B, n = 9).
IL-8 ELISA.
Pulmonary tissue extracts were prepared as described above, and IL-8 was quantified according to protocols provided by the manufacturer (cytokines, R&D Systems; C-reactive protein, Tridelta Development LTD, Wicklow, Ireland). Because of the limited amount of tissue, data from six animals were not obtained (one at baseline, two in group A, and three in group B; baseline, n = 8; A, n = 8; B, n = 7).
Pulmonary histology.
Sections from formalin-fixed lung tissue from the right lower lung lobe were prepared and stained with hematoxylin and eosin according to standard histologic procedures. Slides were microscopically assessed for pathologic change. Only two types of changes were evident: capillary congestion and transudation of fluid into the alveolar spaces. Ten slides in the baseline group and each treatment category were reviewed, and the mean score ± SEM was calculated. The changes were scored blindly on an arbitrary scale from 0 (no change) to 3 (severe) changes.
Statistics.
Statistical analysis was performed by SPSS version 11 (SPSS Inc., Chicago, IL). For studying the relationship among MMP-2, MMP-9, total MMP-activity, IL-8, and histologic changes as dependent variables and O2 and CO2 as independent variables, univariate ANOVA was used. To accommodate for multiple comparisons, p values were adjusted according to Bonferroni. For comparing groups of continuous variables, independent sample t tests were used. The statistical analysis of the in situ zymography data was conducted by ANOVA followed by post hoc t test. All values are given as mean ± SEM. A level of p < 0.05 was considered as statistically significant. The number of 10 piglets in each group was selected on the basis of a compromise between economic resources and the requirements for the statistical models proposed.
RESULTS
Piglets.
There were no significant differences between the groups with respect to body weight, age, sex, rectal temperature, or cardiovascular or biochemical variables at baseline. Characteristics of the hypoxic insult such as hypoxemia time, mean arterial blood pressure, base excess (BE), and pH were similar in the groups as previously reported (21). There were no significant differences in any outcome measures or between different Paco2 levels; hence, the subgroups were combined.
Total MMP activity.
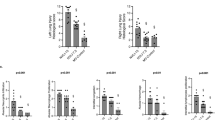
Broad matrix degrading capacity was examined in pulmonary tissue to study whether any differences in total MMP activity could be seen between piglets at baseline and at the end of the experiment. Total MMP activity was significantly increased in group A and nearly 2-fold increased in group B compared with baseline. There were no significant differences between groups A and B (p = 0.2; Fig. 1).
Total MMPs in pulmonary tissue. Broad matrix degrading capacity was examined in pulmonary tissue to study whether any differences in total MMP activity could be seen between the controls at baseline (n = 5) and the piglets at the end of the experiment [group A = 21% O2 (n = 27); group B = 100% O2 (n = 27)]. Total MMP activity was significantly increased in group A [983 ± 62 relative fluorogenic unit (RFU)] and nearly 2-fold increased in group B (1111 ± 80 RFU) compared with baseline (700 ± 83 RFU). There were no significant differences between groups A and B (p = 0.2). Results are presented as mean ± SEM; *p < 0.05, 21% O2 vs baseline; **p < 0.005, 100% O2 vs baseline.
Gelatinolytic activity.
Gel zymography was applied on pulmonary tissue and BAL fluid to determine the activity of MMPs (Fig. 2). Gelatinolytic activity was detected at 92, 72, and 62 kD, corresponding to pro–MMP-9, pro–MMP-2, and active MMP-2, respectively (Fig. 2). In pulmonary tissue extracts, pro–MMP-9, pro–MMP-2, and active MMP-2 were significantly increased in the resuscitated groups compared with baseline (p < 0.001; Fig. 3). Pro– and active MMP-2 were markedly elevated in group B compared with group A (p < 0.005), whereas no significant differences between groups A and B were seen measuring pro–MMP-9 (Fig. 3). In the BAL fluid, there was a 2-fold increase in pro–MMP-9 and pro–MMP-2 in group B compared with group A (p = 0.001 and 0.021, respectively; Fig. 4).
Gelatinolytic activity in pulmonary tissue extracts. (A) Pro–MMP-9 was significantly increased in group A [21% O2 (n = 28)] and group B [100% O2 (n = 28)] compared with baseline (n = 8). Pro– (B) and active (C) MMP-2 were significantly increased in group B (n = 28) compared with group A (n = 28) and baseline (n = 8). Results are presented as mean ± SEM; **p < 0.002.
In situ zymography.
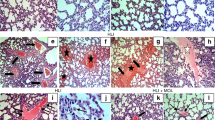
In situ zymography was used to detect the cellular distribution of net metalloproteinase activity resulting from the balance between MMPs and their endogenous inhibitors. Fluorescence intensity was heterogeneously distributed across regions in control lungs with an increasing gradient as follows: alveoli < submucosa < blood vessels. The bronchial epithelium and cartilage stained negatively. As illustrated in Fig. 5, the pattern of in situ zymography differed after resuscitation with 21% O2 or 100% O2. In group A, the levels of fluorescence remained unchanged in alveoli and submucosa compared with baseline lungs, and only a 41% increase was detected in the blood vessels. In group B, a significant increase was detected in both the submucosa (47%) and the blood vessels (50%), whereas no changes were observed in the alveoli. The incubation of sections with the MMP inhibitor phenanthrolene virtually abolished fluorescence in lungs from piglets that were resuscitated with 100% O2, indicating that both constitutive and hypoxia/resuscitation-induced fluorescence were indeed the result of net MMP activity on gelatin.
Net gelatinolytic activity increases in lungs 2.5 h after hypoxia resuscitation. (A) Fluorescence photomicrographs of lung sections showing in situ zymography in baseline piglets (BL) after reoxygenation with 21 or 100% O2. (B) Quantification of gelatinolytic activity in arbitrary units of fluorescence. Note that fluorescence increases in blood vessels (arrows) and submucosa (dots) after reoxygenation with pure O2, whereas the significant increase is detected only in blood vessels in piglets that were resuscitated with 21% O2. In all cases, fluorescence levels in alveoli (arrowheads) remained unchanged. Broad spectrum MMP inhibitor phenanthrolene (Phen) nearly abolished gelatinolytic activity. Values represent the means ± SEM of triplicate assays obtained from five animals per group. Bar = 200 μm.
MMP-2 mRNA expression.
To elucidate further the mechanism behind the augmented MMP activity in pulmonary tissue, we measured the mRNA expression of MMP-2 by quantitative real-time PCR in groups A and B. Expression of MMP-2 mRNA was increased in the resuscitated groups compared with baseline (MMP-2,18S 14.6 ± 0.8 and MMP-2/18S 9.8 ± 0.7, respectively; p < 0.001). We were unable to detect significant differences between groups A and B because of large pig-to-pig variations (p = 0.6).
ORAC
Total antioxidant capacity, noted as ORAC values, was measured in pulmonary tissue extracts in baseline piglets and in groups A2 and B2 (Fig. 6). Compared with baseline (15 ± 1 μmol TE/g tissue), the mean ORAC values of tissue from group A (10.3 ± 0.9 μmol TE per gram tissue), as well as group B (6.6 ± 0.5 μmol TE/g tissue), were significantly reduced (p < 0.005 and p < 0.001, respectively). Furthermore, ORAC values were significantly (p = 0.003) reduced in group B compared with group A.
Pulmonary endogenous antioxidant capacity measured as ORAC at baseline (n = 8) and after resuscitation with 21% O2 (n = 10) or 100% O2 (n = 9). The results are expressed as μmol TE/g wet weight of tissue samples. The ORAC value of tissue from piglets that were resuscitated with 21% O2 or 100% O2 was significantly reduced compared with baseline. Comparing piglets that were resuscitated with 21% O2 and 100% O2, there was a significant lowering of the ORAC value. Results are presented as mean ± SEM, **p < 0.005.
IL-8 level
Compared with baseline (43.75 ± 5 pg/mL), IL-8 concentration in pulmonary tissue nearly doubled in group A (74 ± 114 pg/mL; p < 0.05) and nearly tripled in group B (122 ± 18 pg/mL; p < 0.05; Fig. 7). In addition, IL-8 concentration increased nearly 2-fold in group B compared with group A (p < 0.05).
PULMONARY HISTOLOGY
Only two types of pathologic changes were detected microscopically in conventionally stained slides: capillary congestion and transudation of fluid into the alveolar spaces. In the baseline group, only a light capillary and venous dilation was seen (0.56 on a scale from 0 to 3; see “Methods”; a low degree of congestion regularly occurs post mortem as an agonal phenomenon to death). The level of congestion was not significantly different in the resuscitated groups. However, a low degree of alveolar transudation was present (0.30 on a scale from 0 to 3 in both groups that received 21% and 100% O2). There was no observed effect of different Pco2 levels.
DISCUSSION
The current study concludes that resuscitation of piglets with 100% O2 after global hypoxia significantly increases pulmonary MMP and IL-8 levels and reduces endogenous antioxidant capacity, suggesting that these changes are triggered by oxidative stress. This was in contrast to findings in animals that were resuscitated with 21% O2. To our knowledge, it has not been previously reported that pulmonary MMP-2 and -9 activity increases more after hypoxia-reoxygenation with 100% O2 than with 21% O2. We recently published similar data from studies of the brain and heart in the applied piglet model (21,27). In these studies, we concluded that resuscitation with 100% O2 subsequent to global hypoxia increases cerebral and myocardial injury compared with resuscitation with 21% O2. Increased MMP activity was investigated previously in a hyperoxic piglet pulmonary injury model but only after 72 h of exposure (11). Hypoxia-reoxygenation injury triggers MMP production earlier than just hyperoxia (28). We found differences between the groups of resuscitated piglets as early as 2.5 h after resuscitation. It is interesting that these changes occur so early after hypoxia-reoxygenation, placing MMPs upstream in the pathologic cascade that may lead to a pulmonary damage over time. MMP-2 mRNA levels in pulmonary tissue were significantly increased in resuscitated piglets with respect to baseline. However, there were no significant differences in MMP-2 mRNA between the resuscitated groups, suggesting that the changes found in MMP-2 expression occur at the posttranscriptional level. In line with this, similar changes were found in myocardial tissue (27). In contrast, cerebral MMP-2 mRNA level in this model was significantly higher in piglets that were resuscitated with 100% O2 compared with 21% O2 (21). Therefore, it is possible that a higher level of O2 influences different tissues in various ways.
In situ zymography also showed increased gelatinase activity in the piglets that were resuscitated with 100% O2 compared with 21% O2. After resuscitation with 100% O2, a significant increase was detected in both submucosa and blood vessels, whereas no changes were observed in the alveoli. This cell-dependent profile of MMP activity may rely on a larger buffering capacity of endogenous MMP inhibitors (i.e. TIMPs) in the alveoli than in other areas of the lung, which is consistent with findings in other studies (10). It is also conceivable that ORAC capacity differs across cell types, depending on their proximity to an airway and the O2 concentration that reaches neighboring areas, such as the submucosa. This is supported by the findings in a study that showed increased airway-associated MMP by in situ zymography in a TIMP-3 knockout mice sepsis study (29). Oxidative stress and excess of free radicals in piglets that are resuscitated with pure O2 may contribute to activating MMP-2 and possibly pro–MMP-9 and may account for the differences in total MMP activity and the net gelatinolytic activity observed between normoxic and hyperoxic piglets. Indeed, free radicals have previously been reported to induce gene expression of several MMPs and to activate MMPs posttranslationally (30,31), even in the absence of pro-peptide cleavage (32). The ORAC assay provides significant information regarding the antioxidant capacity of various tissue samples. By measuring a broad spectrum of different types of antioxidant activities over a given time span, it represents a relevant in vivo situation (25). The marked reduction of the ORAC value in piglets that go through resuscitation with 100% O2 indicates less total antioxidant capacity remaining compared with piglets that are resuscitated with 21% O2. We previously published a considerable reduction in cerebral and myocardial ORAC value in piglets that were resuscitated with 100% O2 compared with 21% O2, underscoring the impact of this finding (21,27).
In the present study, we found that IL-8 level in pulmonary tissue was doubled in piglets that were resuscitated with 100% O2 compared with 21% O2. This response was presumably induced by O2 toxicity. In clinical studies, increased levels of proinflammatory cytokines (i.e. IL-8) in BAL fluid correlated with the degree of pulmonary dysfunction and predicted development of chronic lung disease in premature infants (33). Importantly, IL-8 level is known to regulate NF-κB signaling via a redox-sensitive mechanism (34). Therefore, the reduced level of endogenous antioxidant capacity in the 100% O2 group is consistent with the increased IL-8 level.
The present work cannot tell which O2 concentration should be used to resuscitate asphyxiated newborn infants. Animal models will always be an approximation to the clinical situation. It is important, however, to give some considerations to species differences in O2 responses, different biochemical responses, lack of reference values for common functional variables, and different maturation at birth. We used a piglet model because the anatomy and physiology are similar to human (35), but this model is time consuming and it is expensive to obtain large enough series for satisfactory statistical power. It must be emphasized that the animals in the present work were 12–36 h old and, therefore, to some extent adapted to extrauterine life. Thus, whether our findings can be applied to the resuscitation of asphyxiated newborn infants should be settled through clinical trials.
Resuscitation of asphyxiated newborns with 100% O2 has been uncritically accepted for many decades; thus, questioning its effectiveness and safety is difficult. Guidelines from the American Heart Association and the American Academy of Pediatrics recommend that pure O2 should be used during initial newborn resuscitation whenever positive pressure ventilation is required. These guidelines have recently been questioned by us and others (21,27,36). Clinical studies indicate that resuscitation with 21% O2 compared with 100% O2 is safe and results in a quicker recovery (36,37). Further investigations will be required to determine whether 21% O2 resuscitation can improve long-term outcomes. It is possible that adjustment of the fraction of inhaled O2 during resuscitation on the basis of each infant's response, color, and oxyhemoglobin saturation will reduce toxicity to the lungs.
CONCLUSION
In summary, our findings demonstrate that resuscitation of hypoxic piglets with 100% O2 causes more up-regulation of early markers of pulmonary injury than resuscitation with 21% O2. Altogether, these data strongly suggest that caution must be taken when applying pure O2 to newborn infants. We do not know the optimal oxygen concentration during resuscitation, but our data show that 100% O2 seems to be too high in the newborn pig.
Abbreviations
- BAL:
-
bronchoalveolar lavage
- MMP:
-
matrix metalloproteinase
- ORAC:
-
oxygen radical absorbance capacity
- Paco2:
-
arterial carbon dioxide tension
- Pao2:
-
arterial O2 tension
- RT-PCR:
-
reverse transcriptase–PCR
- TE:
-
Trolox equivalent
- TIMP:
-
tissue inhibitor of MMPs
References
Kondo M, Itoh S, Isobe K, Kondo M, Kunikata T, Imai T, Onishi S 2000 Chemiluminescence because of the production of reactive oxygen species in the lungs of newborn piglets during resuscitation periods after asphyxiation load. Pediatr Res 47: 524–527
Askie LM, Henderson-Smart DJ, Irwig L, Simpson JM 2003 Oxygen-saturation targets and outcomes in extremely preterm infants. N Engl J Med 349: 959–967
Fowlie PW, Bancalari E 2005 Not just a lot of hot air for the babies—the air versus oxygen debate needs to be seriously considered. Biol Neonate 87: 35–37
Buisson AC, Zahm JM, Polette M, Pierrot D, Bellon G, Puchelle E, Birembaut P, Tournier JM 1996 Gelatinase B is involved in the in vitro wound repair of human respiratory epithelium. J Cell Physiol 166: 413–426
Legrand C, Gilles C, Zahm JM, Polette M, Buisson AC, Kaplan H, Birembaut P, Tournier JM 1999 Airway epithelial cell migration dynamics. MMP-9 role in cell-extracellular matrix remodeling. J Cell Biol 146: 517–529
Cederqvist K, Sorsa T, Tervahartiala T, Maisi P, Reunanen K, Lassus P, Andersson S 2001 Matrix metalloproteinases-2, -8, and -9 and TIMP-2 in tracheal aspirates from preterm infants with respiratory distress. Pediatrics 108: 686–692
Schock BC, Sweet DG, Ennis M, Warner JA, Young IS, Halliday HL 2001 Oxidative stress and increased type-IV collagenase levels in bronchoalveolar lavage fluid from newborn babies. Pediatr Res 50: 29–33
Speer CP 2004 Pre- and postnatal inflammatory mechanisms in chronic lung disease of preterm infants. Paediatr Respir Rev 5: S241–S244
Ekekezie II, Thibeault DW, Simon SD, Norberg M, Merrill JD, Ballard RA, Ballard PL, Truog WE 2004 Low levels of tissue inhibitors of metalloproteinases with a high Matrix metalloproteinase-9/tissue inhibitor of metalloproteinase-1 ratio are present in tracheal aspirate fluids of infants who develop chronic lung disease. Pediatrics 113: 1709–1714
Dahlin K, Mager EM, Allen L, Tigue Z, Goodglick L, Wadehra M, Dobbs L 2004 Identification of genes differentially expressed in rat alveolar type I cells. Am J Respir Cell Mol Biol 31: 309–316
Gushima Y, Ichikado K, Suga M, Okamoto T, Iyonaga K, Sato K, Miyakawa H, Ando M 2001 Expression of matrix metalloproteinases in pigs with hyperoxia-induced acute lung injury. Eur Respir J 18: 827–837
Woessner JF Jr 1991 Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 5: 2145–2154
Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G 2000 Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood 96: 2673–2681
Li A, Dubey S, Varney ML, Dave BJ, Singh RK 2003 IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol 170: 3369–3376
D'Angio CT, LoMonaco MB, Chaudhry SA, Paxhia A, Ryan RM 1999 Discordant pulmonary proinflammatory cytokine expression during acute hyperoxia in the newborn rabbit. Exp Lung Res 25: 443–465
Groneck P, Gotze-Speer B, Oppermann M, Eiffert H, Speer CP 1994 Association of pulmonary inflammation and increased microvascular permeability during the development of bronchopulmonary dysplasia: a sequential analysis of inflammatory mediators in respiratory fluids of high-risk preterm neonates. Pediatrics 93: 712–718
Speer CP 2001 New insights into the pathogenesis of pulmonary inflammation in preterm infants. Biol Neonate 79: 205–209
Todo G, Herman PG 1986 High-resolution computed tomography of the pig lung. Invest Radiol 21: 689–696
Gehrke I, Pabst R 1990 Cell composition and lymphocyte subsets in the bronchoalveolar lavage of normal pigs of different ages in comparison with germfree and pneumonic pigs. Lung 168: 79–92
Malik AB, Kidd BS 1973 Independent effects of changes in H+ and CO 2 concentrations on hypoxic pulmonary vasoconstriction. J Appl Physiol 34: 318–323
Munkeby B, Borke W, Bjornland K, Sikkeland LI, Borge G, Halvorsen B, Saugstad O 2004 Resuscitation with 100% O2 increases cerebral injury in hypoxemic piglets. Pediatr Res 56: 783–790
Kleiner DE, Stetler-Stevenson WG 1994 Quantitative zymography: detection of picogram quantities of gelatinases. Anal Biochem 218: 325–329
Loy M, Burggraf D, Martens KH, Liebetrau M, Wunderlich N, Bultemeier G, Nemori R, Hamann GF 2002 A gelatin in situ-overlay technique localizes brain matrix metalloproteinase activity in experimental focal cerebral ischemia. J Neurosci Methods 116: 125–133
Rivera S, Ogier C, Jourquin J, Timsit S, Szklarczyk AW, Miller K, Gearing AJ, Kaczmarek L, Khrestchatisky M 2002 Gelatinase B and TIMP-1 are regulated in a cell- and time-dependent manner in association with neuronal death and glial reactivity after global forebrain ischemia. Eur J Neurosci 15: 19–32
Cao G, Prior RL 1999 Measurement of oxygen radical absorbance capacity in biological samples. Methods Enzymol 299: 50–62
Aaby K, Hvattum E, Skrede G 2004 Analysis of flavonoids and other phenolic compounds using high-performance liquid chromatography with coulometric array detection: relationship to antioxidant activity. J Agric Food Chem 52: 4595–4603
Borke WB, Munkeby BH, Halvorsen B, Bjornland K, Tunheim SH, Borge GI, Thaulow E, Saugstad OD 2004 Increased myocardial matrix metalloproteinases in hypoxic newborn pigs during resuscitation: effects of oxygen and carbon dioxide. Eur J Clin Invest 34: 459–466
Ben Yosef Y, Lahat N, Shapiro S, Bitterman H, Miller A 2002 Regulation of endothelial matrix metalloproteinase-2 by hypoxia/reoxygenation. Circ Res 90: 784–791
Martin EL, Moyer BZ, Pape MC, Starcher B, Leco KJ, Veldhuizen RA 2003 Negative impact of tissue inhibitor of metalloproteinase-3 null mutation on lung structure and function in response to sepsis. Am J Physiol Lung 285: L1222–L1232
Okamoto T, Akaike T, Sawa T, Miyamoto Y, van der Vliet A, Maeda H 2001 Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J Biol Chem 276: 29596–29602
Kameda K, Matsunaga T, Abe N, Hanada H, Ishizaka H, Ono H, Saitoh M, Fukui K, Fukuda I, Osanai T, Okumura K 2003 Correlation of oxidative stress with activity of matrix metalloproteinase in patients with coronary artery disease. Possible role for left ventricular remodelling. Eur Heart J 24: 2180–2185
Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA 2002 S-Nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science 297: 1186–1190
Golej J, Winter P, Schoffmann G, Kahlbacher H, Stoll E, Boigner H, Trittenwein G 2002 Impact of extracorporeal membrane oxygenation modality on cytokine release during rescue from infant hypoxia. Shock 20: 110–115
Rahman I 2002 Oxidative stress, transcription factors and chromatin remodelling in lung inflammation. Biochem Pharmacol 64: 935–942
Hannon JP, Bossone CA, Wade CE 1990 Normal physiological values for conscious pigs used in biomedical research. Lab Anim Sci 40: 293–298
Niermeyer S, Vento M 2004 Is 100% oxygen necessary for the resuscitation of newborn infants?. J Matern Fetal Neonatal Med 15: 75–84
Saugstad O, Ramji S, Vento M 2005 Resuscitation of depressed newborn infants with ambient air or pure oxygen: a meta-analysis. Biol Neonate 87: 27–34
Acknowledgements
We thank G. Dyrhaug, J. Lindstad, H. Nilsen, T. Norèn, S. Pettersen, and E.L. Sagen for excellent technical assistance. We are also grateful for valuable advice from G. Aamodt, PhD, and Prof. T. Egeland in the Section of Biostatistics, Rikshospitalet University Hospital, Norway.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was granted by The Norwegian SIDS Society, University of Oslo, AGA AB Medical Research Fund, The Laerdal Foundation for Acute Medicine, and The Norwegian Society of Anaesthesiology. B.H.M. is a Research Fellow at the Faculty Division Rikshospitalet University Hospital, Norway.
Rights and permissions
About this article
Cite this article
Munkeby, B., Børke, W., Bjørnland, K. et al. Resuscitation of Hypoxic Piglets with 100% O2 Increases Pulmonary Metalloproteinases and IL-8. Pediatr Res 58, 542–548 (2005). https://doi.org/10.1203/01.PDR.0000179407.46810.2D
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000179407.46810.2D
This article is cited by
-
Oxygen therapy of the newborn from molecular understanding to clinical practice
Pediatric Research (2019)
-
Transcriptome profiling of the newborn mouse lung after hypoxia and reoxygenation: hyperoxic reoxygenation affects mTOR signaling pathway, DNA repair, and JNK-pathway regulation
Pediatric Research (2013)
-
Brain inflammation induced by severe asphyxia in newborn pigs and the impact of alternative resuscitation strategies on the newborn central nervous system
Pediatric Research (2013)
-
Dynamic FDG PET for assessing early effects of cerebral hypoxia and resuscitation in new-born pigs
European Journal of Nuclear Medicine and Molecular Imaging (2012)
-
Cardio-renal recovery of hypoxic newborn pigs after 18%, 21% and 100% reoxygenation
Intensive Care Medicine (2008)