Abstract
The purpose of the study was to investigate the sequence of processes occurring during and after hypoxia-induced acidemia. We used proton nuclear magnetic resonance spectroscopy, which provides an overview of metabolites in cerebrospinal fluid (CSF), reflecting neuronal metabolism and damage. The pathophysiological condition of acute fetal asphyxia was mimicked by reducing maternal uterine blood flow in 14 unanesthetized pregnant ewes. CSF metabolites were measured during hypoxia-induced acidemia, and during the following recovery period, including the periods at 24 and 48 h after the hypoxic insult. Maximum values of the following CSF metabolites were reached during severe hypoxia (pH ≤ 7.00): glucose, lactate, pyruvate, hypoxanthine, alanine, β-hydroxybutyrate, choline, creatine, myo-inositol, citrate, succinate, valine, and an unknown metabolite characterized by a resonance at 1.56 ppm in the proton nuclear magnetic resonance spectrum. Twenty-four hours after the hypoxic insult, myo-inositol was increased, and alanine was decreased 48 h after the hypoxic insult, both compared with control values. Choline levels in CSF had a linear relationship with arterial pH (r = 0.26, p < 0.005). During severe hypoxia, CSF levels of succinate and choline are increased. Increased CSF levels of succinate may indicate dysfunction of the mitochondrial respiratory chain, whereas elevated CSF choline levels may indicate disrupted cell membranes. The increase of the CSF myo-inositol level after 24 and 48 h may indicate osmolytic cell changes causing cell edema. Decreased alanine level may represent changes in the source of excitatory amino acid synthesis.
Similar content being viewed by others
Main
Neurologic impairment, such as cerebral palsy due to fetal hypoxia, is still an important topic in perinatology. The knowledge of cellular metabolic events that lead to cerebral injury arising from hypoxia is still incomplete. More insight into the sequence of pathologic events may lead to new therapeutic interventions for the fetus and for the newborn to prevent neurologic abnormalities later in life (1).
Lack of oxygen leads to anaerobic metabolism and impaired mitochondrial function, which causes energy failure. Transcellular ion pumps, which depend on sufficient energy, may fail, resulting in cytotoxic edema. ATP is degraded to hypoxanthine, which acts as a substrate for the formation of oxygen-derived free radicals during reoxygenation. In addition to cerebral damage that may occur as a result of these and other complex processes during a hypoxic insult in fetal life, a cascade of events is initiated that triggers neuronal cell injury and death hours or days later. It is suggested that many mechanisms contribute to this secondary energy failure, such as excitotoxin accumulation, NO synthesis, oxygen free radicals, and apoptosis (2). Finally, the combined effects of processes serve to disrupt the structural components of the cell.
When neuronal cell damage has occurred, metabolites are released into the CSF. During ATP depletion, hypoxanthine is released into the CSF (3). Such metabolites can be used as diagnostic biochemical markers of the severity of the hypoxic insult. In asphyxiated neonates, an increase was observed in several CSF metabolites, such as lactate (4), excitatory amino acids, and the amino acids alanine and valine (5). Elevated hypoxanthine levels in CSF were associated with later neurologic disability (6).
1H-NMR spectroscopy is a powerful technique that facilitates an overview of CSF metabolites, representing various metabolic pathways such as glycolysis, TCA cycle, and ATP breakdown. In the present study, we quantified metabolites in CSF of awake fetal sheep, measured using 1H-NMR spectroscopy during normoxia, hypoxia-induced acidemia, recovery, and the days after the hypoxic insult. Furthermore, the concentration of metabolites associated with energy metabolism was related to metabolites associated with structural damage of the cell.
MATRIALS AND METHODS
Surgery.
Fourteen pregnant ewes of the Dutch Texel breed were operated on at a gestational age of 127–128 d (term 147 d). General anesthesia was induced with 25 mg/kg pentobarbital with 0.5 mg atropine i.v. and was maintained in a closed system with 2–3% enflurane in a 2:1 mixture of NO and oxygen. A paramedian abdominal incision was made, after which the uterus was temporarily lifted out of the pelvis and covered with soaked gauze. The peritoneum was opened over the trifurcation of the aorta into the common internal iliac artery and the external iliac arteries. An inflatable balloon occluder (diameter 8 or 10 mm, Rhodes Medical Instruments, Woodland Hills, CA, U.S.A.) was placed around the maternal common internal iliac artery.
The fetal sheep was approached by hysterotomy above the head. Electrodes were implanted s.c. at the level of the cardiac apex and the shoulders to record the fetal ECG. A polyvinyl sample catheter (outer diameter 1.6 mm, inner diameter 0.8 mm) was inserted via the left axillary artery to facilitate sampling of arterial preductal blood. A perivascular ultrasonic blood flow transducer (3SL, S or B, Transonic Systems Inc., Ithaca, NY, U.S.A.) was placed around the left common carotid artery. Amniotic fluid was used to optimize contact of the transducer with the carotid artery. To reach the sagittal sinus, the scalp was incised and the periostium spread aside. A 14-gauge Venflon needle was used to make a hole through the bone above the sagittal sinus at the site where the lamboid suture intersects the sagittal sinus. A polyvinyl catheter (outer diameter 1.6 mm, inner diameter 0.8 mm) was then placed in the sagittal sinus. A rubber disc was fixed at the end of the catheter and was glued to the skull. A midline incision was made in the dorsal surface of the fetal neck to facilitate the CSF sampling. Muscles were spread aside until the dura was clearly visible. The spinal cavity was opened with the tip of a 14-gauge Venflon needle. A polyvinyl catheter (outer diameter 1.6 mm, inner diameter 0.8 mm, with a thickening at 10–15 mm from the tip and two holes in between the tip and the thickening) was inserted through the puncture hole into the spinal cavity until satisfactory CSF sampling was possible. The catheter was sutured to the surrounding tissue. Muscles and skin were closed, and the catheter was sutured to the fetal skin to prevent it being pulled out by traction. A catheter was left in the uterus to measure IUP. All electrodes and catheters were exteriorized through an incision in the ewe's flank and packed into a pouch on the ewe's back. Before the skin was closed, 106 U penicillin was dispersed into the abdomen. The animals were allowed to recover for 4 d after surgery. On the day of operation and during the recovery period, antibiotics were administered daily: depomycine (Intervet, Boxmeer, The Netherlands) intramuscularly to the ewe, amoxicillin CF 125 mg i.v. to the fetus, and amoxicillin CF 125 mg into the amniotic cavity. Fetal blood sample catheters were kept open by continuous infusion of heparinized saline solution (5 IU/mL at 1 mL/h). Carotid flow, ECG, and FHR were measured continuously and Sao2 and Svo2, acid-base balance, and Hb were measured each day to assess the fetal condition during the recovery period before the start of the experiments. Food and water were supplied ad libitum after surgery.
The experiments were approved by the local ethical committee for animal research.
Experiments.
After a control period of 2 h, uteroplacental blood flow was reduced by gradually occluding the common iliac artery to achieve stepwise hypoxia-induced fetal arterial pH levels between 7.23 and 7.27 (mild hypoxia), and finally 7.00 or lower (severe hypoxia), after which the occluder was released. During the time that CSF was collected during hypoxia, the intention was to maintain the hypoxia-induced acidemia at a constant level. Figure 1 shows an example of an experiment on one fetal sheep. Fetal arterial and venous blood samples for blood gases, pH, and Hb (0.2 mL each sample) were taken at 30-min intervals during the control period, and every 5–10 min during hypoxia. During the recovery phase, fetal blood samples were taken every 15 min during the first 2 h. Over the following days, fetal blood samples were taken before and after CSF sampling at 24 and 48 h during recovery. Arterial blood from the axillary artery and venous blood from the sagittal sinus were drawn simultaneously. During the experiments, fetal ECG, FHR, FBP, and carotid BF were measured continuously. CSF (1 mL) and arterial (2.4 mL) and venous blood (2.4 mL) were sampled at the following moments during the experiment: at the end of the control period, during the hypoxic period when an arterial pH of 7.23–7.27 was reached (mild hypoxia), and at a pH of 7.00 or lower (severe hypoxia), and 1, 2, 24, and 48 h after recovery. Blood samples were analyzed for glucose, lactate, purines, and uridine. In the recovery period, lactate and glucose in the blood were only measured at 1 h after recovery. Total blood loss was estimated to be within 14% of the total blood volume. FBP and amniotic pressure were measured at the level of the ewe's back.
Example of an experiment on a fetal sheep. Arterial Sao2 (♦) and pH (□) are plotted against time (min). At t = 0, maternal fraction of inspired oxygen (Fio2) was decreased. The time points when CSF was sampled are indicated by an arrow: control (C), mild hypoxia (MH), severe hypoxia (SH), and 1 (R1), 2 (R2), 24 (R24), and 48 (R48) h after release of the occluder during recovery.
Data analysis.
Fetal blood samples were analyzed within 2 min to assess Sao2, Cao2, pH, BEecf, Hb, and blood gases. Cao2 and BEecf were calculated according to the formula used by the blood gas analyzer (ABL 510, Radiometer, Copenhagen, Denmark). Cerebral oxygen extraction was calculated by dividing Cavo2 by Cao2. Cerebral oxygen consumption was calculated by multiplying the Cavo2 by the carotid BF. For glucose and lactate measurements, fetal blood was collected in special syringes, stored on ice, and measured within 1.5 h. Arterial and venous lactate were measured in whole blood by a spectrophotometric method (Cobas Mira Plus analyzer, Roche Diagnostics Systems, IN, U.S.A.). Glucose was measured in whole blood by means of an enzymatic colorimetric test (SYS 3 BM/Hitachi 747, Roche Molecular Biochemicals, Mannheim, Germany). In plasma, levels of hypoxanthine, xanthine, and inosine were determined in plasma using a HPLC procedure with a reversed-phase column (Alltima C18, Alltech Associates, Deerfield, IL, U.S.A.) and a Millenium 32 Data system (Millipore-Waters, Marlborough, MA, U.S.A.), after centrifugation of the blood at 3000 revolutions/min for 10 min and freezing to −80°C. Disposable transducers with an open-tip catheter placed in the left axillary artery were used to determine FBP. The continuous signals, together with the continuous flow signal from the left carotid artery, were stored on a personal computer using a data acquisition program (Poly Physiologic Analysis Package, Inspektor Research System, Amsterdam, the Netherlands).
The CSF samples (0.25–1.0 mL) were immediately centrifuged (10 min at 3000 revolutions/min) and stored at −80°C. The whole CSF volume was needed for the NMR measurements. Pretreatment of the CSF samples for NMR measurements has been described by Wevers et al.(7). All samples were deproteinized by filter centrifugation.
NMR measurements.
1H-NMR spectroscopy of CSF samples was carried out along the lines described previously (7) on a 600-MHz Bruker spectrometer (Bruker, Newark, DE, U.S.A.). The temperature during the measurements was 25°C, and NMR tubes with a diameter of 5 mm were used. CSF sample volume varied between 100 and 700 μL. The sample volume was filled up to 700 μL with water. For the 1H-NMR measurements, a 60° radiofrequency pulse was used. The delay between two successive pulses was 6 s. The number of scans for each experiment was 128. The water resonance was presaturated during the relaxation delay. The FID was recorded in 33 K data points with a sweep width of 7184 Hz.
NMR data analysis.
A Sine-Bell squared filter was used. The FID were Fourier-transformed after the FID was zero filled to 66 K data points. The chemical shift of the internal standard TSP was set at a position of 0 ppm. To analyze the NMR spectra, we used 1D WinNMR and Winfit software (Bruker Analytische Messtechnik, Karlsruhe, Germany). The phase and baseline of the spectra were corrected manually. Resonances in the spectra were fitted semi-automatically to a Lorentzian line shape model function in the frequency domain. Quantitative data were obtained by calculating metabolite concentrations from the area of its corresponding resonance(s) with respect to the area of the internal standard TSP.
The pH of the CSF influences the chemical shift of some metabolites. Therefore, the pH of the samples was standardized (pH 2.50 ± 0.05).
Statistics.
Measured and calculated fetal blood variables were analyzed by paired t test. Values are expressed as mean ± SD. CSF values were analyzed with Wilcoxon matched-pairs signed-ranks test. The median (range) is given for the CSF metabolites. p Values ≤ 0.05 were considered significant. Linear regression analysis was performed relating arterial pH and lactate concentration to CSF metabolite levels. CSF metabolite levels of the energy metabolism (lactate, hypoxanthine, succinate) were correlated to metabolites of cell damage (choline, hypoxanthine).
RESULTS
Surgery was performed in 19 pregnant ewes; two fetuses died after surgery, in one fetus the arterial catheter was occluded, and in two fetuses we failed to achieve adequate metabolic acidosis, probably due to a malfunctioning occluder. Therefore, a total of 14 fetal sheep were analyzed; CSF was sampled in nine, and venous blood was sampled in 11 fetal sheep. Clotted venous and CSF catheters caused variable number of samples collected at each time point. The number of samples achieved during the various measurement points are set out in the tables. Two of the fetal sheep included in the tables died during severe hypoxia, and at 24 h of recovery, respectively. The remaining 12 fetal sheep were alive 48 h after the hypoxic period. The duration of the hypoxic period varied between 73 and 240 min.
Measured and calculated variables are shown in Table 1. Sao2 decreased from 57.2% during the control period to 23.8% at the end of hypoxia; arterial pH decreased from 7.39 to 6.97, arterial BEecf decreased from −2.4 to −17.8 mM. Sao2, pH, and BEecf were returned to control values after 24 h of recovery. During severe hypoxia, Pco2 was increased and then returned to control levels after 2 h of recovery; Pco2 was slightly elevated after 24 h of recovery. Hb concentrations of the fetal arterial blood were slightly increased during severe hypoxia, and decreased after 2 h of recovery, compared with control values. FHR was increased after 1 h of recovery compared with control values. FBP and the blood flow of the left carotid artery were increased during mild hypoxia, the flow reached maximum values of 101 ± 22 mL/min at a pH of 7.13 ± 0.11. Cerebral oxygen consumption was decreased during mild hypoxia by 52%, and during severe hypoxia by 65%, compared with control values. Cerebral oxygen consumption returned to control values in the recovery period.
Figure 2 shows parts of 1H-NMR spectra of CSF samples obtained from one fetal sheep during control, severe hypoxia, after 2 h of recovery, and after 48 h of recovery.
Parts of 1H-NMR spectra of CSF from one fetal sheep during control (C), severe hypoxia (SH), and after 2 (R2) and 48 (R48) h of recovery. Part of the multiplet of glutamine is shown. DMA, dimethylamine;DMG, dimethylglycine;3.14 s, unknown metabolite at 3.14 ppm, the peak area (compared with the internal standard TSP) did not change significantly during hypoxia.
Fetal CSF concentrations are presented in Table 2. During severe hypoxia and/or after 2 h of recovery, the following metabolites were significantly increased: glucose, lactate, pyruvate, alanine, β-hydroxybutyrate, hypoxanthine, choline, creatine, myo-inositol, citrate, succinate, valine, and the unknown metabolite that is present in the spectrum as a singlet at 1.56 ppm. The values of the metabolites during mild hypoxia (n = 3) fell between the values during normoxia and severe hypoxia (lactate, 7.2–9.9 mM; choline, 17–27 μmol/L). CSF metabolite concentrations after 1 h of recovery (n = 4), were in the same range compared with 2 h after recovery. CSF hypoxanthine after 1 h of recovery was present in two (18 and 76 μmol/L) of the four animals.
After 24 h of recovery, myo-inositol was increased in CSF, whereas alanine was decreased, compared with control values (Table 2). After 48 h of recovery, alanine was decreased compared with control values.
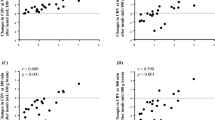
The CSF levels of choline and succinate at the different sample moments for each individual fetal sheep are plotted in Figure 3. The highest levels of succinate were reached during severe hypoxia, and the highest levels of choline after 2 h of recovery.
Table 3 shows the arterial blood concentrations of glucose, lactate, the purines, and the pyrimidine nucleoside uridine. Lactate and hypoxanthine were elevated significantly during mild hypoxia, and increased further during severe hypoxia. After 1 h during recovery, lactate was still elevated, whereas hypoxanthine already was decreasing, and returned to control values after 24 h.
The arteriovenous difference of uridine (Table 4) was significantly increased during mild hypoxia, indicating a cerebral influx of uridine. The arteriovenous difference of lactate was increased after 1 h of recovery, indicating a cerebral lactate influx.
Choline levels in CSF were linearly related to lactate levels in CSF (r = 0.68, p < 0.0001) (Fig. 4), lactate levels in arterial blood (r = 0.49, p < 0.001), and arterial pH (r = 0.26, p < 0.005). The unknown metabolite at 1.56 ppm was correlated with lactate in CSF (r = 0.52, p < 0.0001).
DISCUSSION
The pathophysiological condition of acute fetal asphyxia was mimicked by reducing uterine blood flow in pregnant ewes. Many CSF metabolite levels, which may be related to cerebral cell damage, were elevated during severe hypoxia and the following recovery period. During the days after the hypoxic insult, when oxygenation was returned to control levels, concentrations of metabolites associated with osmoregulation and the production of excitatory amino acids were altered.
Adaptive mechanisms were activated during mild hypoxia, such as increased carotid BF, to maintain cerebral oxygen supply. However, cerebral O2 consumption decreased by 52%. Other experiments in fetal sheep demonstrated that a fall in cerebral oxidative metabolism to <50% of normoxic control values is only noted as arterial pH falls below 7.00 (8). CSF metabolite levels were not changed significantly during mild hypoxia in the three fetuses that could be sampled at this stage in the experiment. In blood, lactate and hypoxanthine concentrations were increased, indicating that systemic anaerobic glycolysis and ATP degradation to hypoxanthine in the heart, liver, and muscles had already started (9). A significantly increased arteriovenous difference for uridine suggests an increased cerebral influx of uridine during mild hypoxia. Uridine uptake in nerve cells may be an expression of neuronal RNA synthesis (10).
The situation during severe hypoxia, accompanied with severe metabolic acidosis (pH < 7.00), may be associated with histologic damage (11). However, FBP, which is an important factor in the development of cerebral damage, was not reduced during the experiment. Similar experiments with chronically instrumented fetal sheep showed minimal histologic changes in cerebellar Purkinje cells, and edematous cell changes (12). With ongoing hypoxia, anaerobic metabolism develops further, pyruvate is converted to lactate, causing acidosis. Alanine, formed from pyruvate by a transamination reaction, has been reported to increase during hypoxia in the extracellular space in the cerebral cortex of fetal sheep (13). Lactate and alanine were increased during severe hypoxia in the CSF of the fetal sheep. Severe hypoxia, with decreased cerebral oxygen consumption of 65%, was accompanied by pathologic CSF lactate levels of 10 mM and higher, which is associated with low Apgar scores in asphyxiated neonates (4).
In our previous study in anesthetized fetal sheep (14), CSF levels of lactate, alanine, hypoxanthine, and choline were also significantly increased during severe hypoxia. An important difference between the two studies is that the FBP in the present study did not decrease, as it did in the previous study during severe hypoxia. Consequently, CSF lactate concentration was higher during severe hypoxia in our previous work, which implies that there was increased cerebral anaerobic metabolism in the experiments performed during anesthesia and surgery. CSF levels of hypoxanthine, choline, and myo-inositol were higher both during control and severe hypoxia in the fetal sheep with anesthesia and surgery, which implies that anesthesia and surgery such as CSF punctures during the experiments have influence on the CSF metabolites.
Succinate couples the TCA cycle to the respiratory chain, because succinate dehydrogenase is directly linked to the electron transport chain. During hypoxia, succinate cannot be converted, and accumulation of succinate is expected. A decrease in flux through the TCA cycle occurs in severe hypoxia, as shown in a 13C-NMR spectroscopic study of cerebral-cortex extracts (15). In the present experiments, succinate was increased in CSF during severe hypoxia.
Lack of oxygen impairs oxidative phosphorylation, leading to reduced ATP production. Energy-consuming ion pumping to maintain membrane potential fails, and Ca2+ enters the cell, which is central to cell death (16). A water influx accompanies the cellular influx of Ca2+ and Na+, leading to cytotoxic cerebral edema. Myo-inositol is one of the major organic intracellular osmolytes in the brain (17), which protect cells from the perturbing effects of osmotic changes. Myo-inositol in CSF was increased during severe hypoxia; this may be caused by a cellular efflux of myo-inositol, being present in high concentrations in the cell, when osmotic regulation fails. Finally, the combined effects of processes serve to disrupt the structural components of the cell. Studies in rats showed an increase in cerebral extracellular choline concentration after ischemia or hypoxia (18). Choline, a component of cell membranes, was elevated during severe hypoxia and in the recovery phase in CSF, indicating cell membrane breakdown.
During the recovery phase in the hours after the hypoxic insult, Sao2 increased, the metabolic acidosis diminished, and cerebral oxygen consumption reached control values. After 2 h of recovery, the Sao2 and pH of the arterial blood were still decreased compared with control. At this point of recovery, a cerebral influx of lactate was present, as was demonstrated earlier in anesthetized fetal sheep by Thiringer et al.(19). This may indicate the use of lactate as an alternative fuel in addition to glucose during recovery. Although after 1 h of recovery the cerebral Sao2 consumption is normalized, the CSF metabolites reach the highest concentrations, as was also shown by de Haan et al.(20). During severe hypoxia, ATP is degraded to hypoxanthine, producing oxygen free radicals during reoxygenation in the recovery phase (6). Hypoxanthine was elevated during severe hypoxia, and present (above detection levels) during recovery in half the animals.
A period of secondary energy failure, despite adequate oxygen and substrate, is characterized by a secondary reduction in cerebral phosphorylation potential, hours to days after the hypoxic insult, as shown by decreasing phosphocreatine/inorganic phosphate ratio in neonates (21) and piglets (22). Secondary cell damage is clinically important because it correlates with residual effects; the greater the extent of the secondary cerebral energy impairment, the worse the neurodevelopmental impairments in human neonate survivors (23). Furthermore, experimental pharmacological intervention with scavengers of free oxygen radicals in the stage after the acute hypoxic period reduced cerebral damage (24). Secondary edema and postinsult epileptiform activity occur hours after the insult, with necrosis of the cortex in fetal sheep (25). Myo-inositol, one of the major organic osmolytes in the brain, is accumulated into cells through a Na+/Myo-inositol co-transporter to protect the cell from osmotic challenges. The expression of this cotransporter mRNA was increased 2 d after middle cerebral artery occlusion in rats (17). In the present study, myo-inositol was increased again 24 h after severe hypoxia, compared with control values in the CSF of the fetal sheep. Failure of the co-transporter, possibly due to the decreased available energy present during secondary energy failure, may result in edema.
Excitatory amino acids are thought to contribute to cerebral cell damage during and after hypoxia. Tan et al.(13) showed that the secondary rise in cortical glutamate corresponded to that of the seizure activity in fetal sheep. Investigations using cultured cells have suggested that alanine might act as a neuronal precursor, producing glutamate via alanine aminotransferase (EC 2.6.1.2) under conditions when the glutamine pool becomes depleted (26). Visual observation of the spectra shows decreased glutamine during secondary recovery, compared with severe hypoxia (Fig. 2). However, Griffin et al.(27) showed in brain slices that alanine is not a significant precursor of glutamate, but does generate the excitatory amino acid aspartate. Although 1H-NMR spectroscopy is not sensitive enough to detect the concentrations of glutamate or aspartate, a decreased CSF alanine concentration was detected 24 and 48 h after hypoxia, compared with control values in the fetal sheep. This suggests that alanine might be used for the production of glutamate during secondary energy failure.
An existing relation between blood parameters used to diagnose fetal hypoxia such as pH, and CSF metabolites indicating cerebral damage, will help to ascertain whether brain damage may have occurred. The fact that choline levels in CSF are better correlated with arterial lactate concentrations than with arterial pH indicates that lactate measurements during and after labor might be preferable above pH measurements.
Elevation of the unknown metabolite present at a resonance of 1.56 ppm indicates that other interesting metabolites play a role in cerebral metabolism during hypoxia. This metabolite level increased with lactate levels in CSF. Unfortunately, the metabolite has not yet been identified.
In conclusion, 1H-NMR spectroscopy provides an overview of known and unknown metabolites in CSF reflecting cerebral metabolism during hypoxia and secondary energy failure. During severe hypoxia, increased succinate reflects a disturbed respiratory chain. Choline release may indicate cerebral cell membrane damage. During recovery, 1–2 h after severe hypoxia, most metabolites such as choline reached the highest concentration in the CSF, whereas hypoxanthine as substrate to produce dangerous oxygen free radicals during re-oxygenation, is still present. One day after the hypoxic period, a CSF myo-inositol level increase may indicate osmolytic cell changes reflecting cell edema, whereas 2 d after the hypoxic period, decreased alanine may represent changes in the source of excitatory amino acid synthesis. Lactate in blood is correlated with choline in CSF, and therefore lactate measurements in clinical use should regain interest.
Abbreviations
- BEecf:
-
extracellular fluid base excess
- carotid BF:
-
carotid blood flow
- Cao2:
-
arterial oxygen content
- Cvo2:
-
venous oxygen content
- Cavo2:
-
arteriovenous difference in oxygen content
- CSF:
-
cerebrospinal fluid
- FBP:
-
fetal blood pressure
- FHR:
-
fetal heart rate
- FID:
-
free induction decay
- 1H-NMR:
-
proton nuclear magnetic resonance
- IUP:
-
intrauterine pressure
- NO:
-
nitric oxide
- Sao2:
-
arterial oxygen saturation
- Svo2:
-
venous oxygen saturation
- TCA:
-
tricarboxylic acid
- TSP:
-
trimethylsilyl-2,2,3,3-tetradeuteropropionic acid
References
van Bel F, Shadid M, Moison RM, Dorrepaal CA, Fontijn J, Monteiro L, Van de Bor M, Berger HM 1998 Effect of allopurinol on postasphyxial free radical formation, cerebral hemodynamics, electrical brain activity. Pediatrics 101: 185–189
Beilharz EJ, Williams CE, Dragunow M, Sirimanne ES, Gluckman PD 1995 Mechanisms of delayed cell death following hypoxic-ischemic injury in the immature rat: evidence for apoptosis during selective neuronal loss. Brain Res Mol Brain Res 29: 1–14
Harkness RA 1988 Hypoxanthine, xanthine uridine in body fluids, indicators of ATP depletion. J Chromatogr 429: 255–278
Mathew OP, Bland H, Boxerman SB, James E 1980 CSF lactate levels in high risk neonates with without asphyxia. Pediatrics 66: 224–227
Hagberg H, Thornberg E, Blennow M, Kjellmer I, Lagercrantz H, Thiringer K, Hamberger A, Sandberg M 1993 Excitatory amino acids in the cerebrospinal fluid of asphyxiated infants: relationship to hypoxic-ischemic encephalopathy. Acta Paediatr 82: 925–929
Harkness RA, Lund RJ 1983 Cerebrospinal fluid concentrations of hypoxanthine, xanthine, uridine adenosine: high concentrations of the ATP metabolite, hypoxanthine, after hypoxia. J Clin Pathol 36: 1–8
Wevers RA, Engelke U, Wendel U, de Jong JG, Gabreëls FJ, Heerschap A 1995 Standardized method for high-resolution 1H-NMR of cerebrospinal fluid. Clin Chem 41: 744–751
Richardson BS, Carmichael L, Homan J, Patrick JE 1993 Cerebral oxidative metabolism in fetal sheep with prolonged graded hypoxemia. J Dev Physiol 19: 77–83
Thiringer K, Karlsson K, Rosen KG, Kjellmer I 1984 Contribution of heart muscle, liver, skeletal muscle placenta to the asphyxial hypoxanthine elevation in the acutely exteriorised fetal lamb. Biol Neonate 45: 169–182
Fog R 1992 Neuronal uridine metabolism. Acta Neurol Scand Suppl 137: 45–47
Low JA 1993 The relationship of asphyxia in the mature fetus to long-term neurologic function. Clin Obstet Gynecol 36: 82–90
De Haan HH, van Reempts JL, Vles JS, de Haan J, Hasaart TH 1993 Effects of asphyxia on the fetal lamb brain. Am J Obstet Gynecol 169: 1493–1501
Tan WK, Williams CE, During MJ, Mallard CE, Gunning MI, Gunn AJ, Gluckmann PD 1996 Accumulation of cytotoxins during the development of seizures edema after hypoxic-ischemic injury in late gestation fetal sheep. Pediatr Res 39: 791–797
Van Cappellen van Walsum AM, Jongsma HW, Wevers RA, Nijhuis JG, Crevels J, Engelke UFH, Moolenaar SH, Oeseburg B, Nijland R 2001 Hypoxia in fetal sheep: a study with1H-NMR spectroscopy of cerebrospinal fluid. Pediatr Res 49: 698–704
Ben-Yoseph O, Badar Goffer RS, Morris PG, Bachelard HS 1993 Glycerol 3-phosphate lactate as indicators of the cerebral cytoplasmic redox state in severe mild hypoxia respectively: a 13C- 31P-n.m.r. study. Biochem J 291: 915–919
Kristian T, Siesjo BK 1996 Calcium-related damage in ischemia. Life Sci 59: 357–367
Yamashita T, Kohmura E, Yamauchi A, Shimada S, Yuguchi T, Sakaki T, Miyai A, Tohyama M, Hayakawa T 1996 Induction of Na+/myo-inositol cotransporter mRNA after focal cerebral ischemia: evidence for extensive osmotic stress in remote areas. J Cereb Blood Flow Metab 16: 1203–1210
Scremin OU, Jenden DJ 1989 Focal ischemia enhances choline output decreases acetylcholine output from rat cerebral cortex. Stroke 20: 92–95
Thiringer K, Blomstrand S, Hrbek A, Karlsson K, Kjellmer I 1982 Cerebral arterio-venous difference for hypoxanthine lactate during graded asphyxia in the fetal lamb. Brain Res 239: 107–117
De Haan HH, Yzermans AC, de Haan J, Van Belle H, Hasaart TH 1994 Effects of surgery asphyxia on levels of nucleosides, purine bases, lactate in cerebrospinal fluid of fetal lambs. Pediatr Res 36: 595–600
Hope PL, Costello AM, Cady EB, Delpy DT, Tofts PS, Chu A, Reynolds EOR 1984 Cerebral energy metabolism studied with phosphorus NMR spectroscopy in normal birth-asphyxiated infants. Lancet 2: 366–370
Lorek A, Takei Y, Cady EB, Wyatt JS, Penrice J, Edwards AD, Peebles D, Wylezinska M, Owen-Reece H, Kirkbride V, Cooper DE, Aldridge RF, Roth SC, Brown G, Delpy DT, Reynolds EOR 1994 Delayed (“secondary”) cerebral energy failure after acute hypoxia-ischemia in the newborn piglet: continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. Pediatr Res 36: 699–706
Roth SC, Baudin J, Cady E, Johal K, Townsend JP, Wyatt JS, Reynolds EO, Stewart AL 1997 Relation of deranged neonatal cerebral oxidative metabolism with neurodevelopmental outcome head circumference at 4 years. Dev Med Child Neurol 39: 718–725
Thordstein M, Bågenholm R, Thiringer K, Kjellmer I 1993 Scavengers of free oxygen radicals in combination with magnesium ameliorate perinatal hypoxic-ischemic brain damage in the rat. Pediatr Res 34: 23–26
Williams CE, Gunn A, Gluckman PD 1991 Time course of intracellular edema epileptiform activity following prenatal cerebral ischemia in sheep. Stroke 22: 516–521
Erecineska M, Nelson D, Nissim I, Daikhin Y, Yudkoff M 1994 Cerebral alanine transport alanine aminotransferase reaction: alanine as a source of neuronal glutamate. J Neurochem 62: 1953–1964
Griffin JL, Rae C, Dixon RM, Radda GK, Matthews PM 1998 Excitatory amino acid synthesis in hypoxic brain slices: does alanine act as a substrate for glutamate production in hypoxia?. J Neurochem 71: 2477–2486
Acknowledgements
The authors thank Theo Arts and Alex Hanssen of the Central Animal Laboratory Nijmegen, and Sjaak van Asten for their assistance. 1H-NMR spectra were recorded at the Dutch hf-NMR facility at the Department of Biophysical Chemistry, University of Nijmegen, The Netherlands (department head, Prof. C.W. Hilbers). We also thank J. Joordens for his invaluable help and assistance. Furthermore, we gratefully acknowledge the help of K. Wethly and A. Stegeman of the Laboratory for Pediatrics and Neurology for the measurement of the blood samples for purines.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by Nellcor Puritan Bennett Inc., Pleasanton, CA, U.S.A.
Rights and permissions
About this article
Cite this article
Van Cappellen Van Walsum, AM., Jongsma, H., Wevers, R. et al. 1H-NMR Spectroscopy of Cerebrospinal Fluid of Fetal Sheep during Hypoxia-Induced Acidemia and Recovery. Pediatr Res 52, 56–63 (2002). https://doi.org/10.1203/00006450-200207000-00012
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200207000-00012
This article is cited by
-
Simultaneous determination of lactic acid and pyruvic acid in tissue and cell culture media by gas chromatography after in situ derivatization-ultrasound-assisted emulsification microextraction
Analytical and Bioanalytical Chemistry (2019)
-
Urine metabolomic profiling of children with respiratory tract infections in the emergency department: a pilot study
BMC Infectious Diseases (2016)
-
Comprehensive 1H NMR metabolic profiling of body fluids for differentiation of meningitis in adults
Metabolomics (2016)
-
Propofol Compared with Isoflurane Inhibits Mitochondrial Metabolism in Immature Swine Cerebral Cortex
Journal of Cerebral Blood Flow & Metabolism (2014)
-
Dicholine salt of succinic acid, a neuronal insulin sensitizer, ameliorates cognitive deficits in rodent models of normal aging, chronic cerebral hypoperfusion, and beta-amyloid peptide-(25–35)-induced amnesia
BMC Pharmacology (2008)