Abstract
Previous findings that the intermediate filament nestin is expressed in immature skeletal muscle cells prompted us to compare the staining patterns of nestin and desmin in rhabdomyosarcomas (RMSs) and in other small cell tumors of infancy. We found that nestin immunoreactivity was present in all of 29 examined typical RMSs, which also expressed desmin. Two undifferentiated tumors, primarily suspected to be RMSs, expressed nestin, but not desmin. One of these nestin-positive, desmin-negative tumors was positive for the expression of the myogenic regulatory gene MyoD and is considered to represent an undifferentiated RMS. The other, a paratesticular tumor, did not contain transcripts for MyoD, and most likely does not represent a RMS. In several RMSs and nonmuscle tumors, a z-disc-associated nestin immunoreactivity occurred as a paramalignant phenomenon in cross-striated muscle fibers adjacent to the tumor cells. Our findings indicate that nestin, although present also in tumors of the central and peripheral nervous systems, as well as in endothelial cells and in some muscle cells adjacent to tumors, is a useful complementary marker for RMS, particularly in very undifferentiated desmin-negative tumors.
Similar content being viewed by others
Main
RMS, the most common soft tissue sarcoma in childhood, accounts for 4-8% of all malignant diseases in children up to 15 y of age. It is presumed to arise from the same embryonic mesenchyme that forms striated skeletal muscle. RMS can be divided into two major groups, embryonal and alveolar RMSs, which represent two distinct tumor subtypes differing in underlying genetic changes, histopathology, predominant localization, and malignancy(1). At present, histologic diagnosis of both embryonal and alveolar RMS is based on classical morphologic criteria, identification of rhabdomyoblasts with or without cross-striation, and immunohistochemical identification of muscle specific proteins, e.g. α-actin and the intermediate filament desmin(2).
IFs have proven very useful for tumor diagnostic purposes, mainly because their expression pattern shows a high degree of tissue specificity, and the IF proteins are stable and easy to analyze in clinical practice. For RMS diagnosis, the class III IF desmin is generally considered a sensitive marker(3, 4).
However, poorly differentiated RMS tumors commonly constitute a diagnostic problem. Such tumors lack rhabdomyoblasts and sometimes also established muscle markers including desmin, and thus are difficult to distinguish from other small cell tumors(5). The characteristics of nestin, a more recently characterized IF(6), suggests that it may serve as a complementary marker for poorly differentiated RMSs. During early embryogenesis nestin is found in somites and in neuroectodermal stem cells(6, 7). Later during development, nestin is transiently expressed at high levels in the myoblast lineage and in CNS stem cells(6, 8). In postnatal skeletal muscle cells, nestin is down-regulated concomitant with an up-regulation of desmin. Undifferentiated myoblasts also express vimentin, an IF found in a variety of mesenchymal cell types. Although nestin belongs to a different class of IFs than vimentin and desmin, it appears to contribute to the same filament formations as the other two in undifferentiated myoblasts and young muscle fibers(9).
Based on the normal developmental expression of nestin in muscle progenitor cells and immature muscle, we set out to investigate the expression of nestin, and desmin for a comparison, in 42 tumors of muscle and non-muscle origin. Twenty-nine of these were embryonal or alveolar RMS. Two were undifferentiated tumors presumed to be RMS, and the remainder were small cell tumors of non-muscle origin. Expression of the myogenic regulatory gene MyoD was analyzed in nestin-positive tumors of unknown origin, to establish a possible myogenic origin. The potential use of immunohistochemical detection of nestin for detection of RMSs is discussed.
METHODS
Preparation of tissue sections. Tumor biopsies, and the lower limb of an electively aborted human fetus of 10-wk gestational age, were fixed in 4% formaldehyde solution and embedded in paraffin. The tissues were cut into 4-6-μm thick sections, dried for 2 h at 48 °C, and stored at room temperature until further processed.
Tumor classification. The tumors were classified according to the SIOP(2, 10). The material consisted of 29 unselected RMSs from various locations (urinary bladder, prostate, extremities, liver, pleura, nasal wing, paratesticular), two embryonal sarcomas, one embryonal ovarian carcinoma, two Ewing's tumors, one neuroblastoma, one non-Hodgkin lymphoma, one peripheral PNET, one lung metastasis from a small cell tumor of unknown origin, one fibrosarcoma, one ganlioneuroblastoma, one Wilms' tumor with rhabdomyomatous differentiation, and one ektomesenchymoma (see Table 1).
Antibodies. The anti-nestin antiserum no. 130 was produced in a rabbit immunized with a bacterially produced fusion protein containing the 1300 carboxy-terminal amino acid residues of the rat nestin protein. The mouse monoclonal antidesmin antibodies (D33) were purchased from Dako. Specificities of the antibodies used have been documented previously(8, 11, 12). In immunoblotting, the anti-nestin serum no. 130 reacts predominantly with a band of approximately 200 kD, the expected molecular weight of nestin(8).
Immunohistochemistry. Tissue sections were deparaffinized in xylene, three times for 10 min each, rehydrated, and treated with 0.7% hydrogen peroxide in methanol for 25 min, followed by 3% BSA in PBS for 30 min. Consecutive sections were incubated for 2 h at room temperature with the anti-nestin serum no. 130 diluted 1:1000 in PBS, or mouse monoclonal anti-desmin antibodies (D33, Dako) diluted 1:200 in PBS, followed by 1 h of incubation with biotinylated swine anti-rabbit immunoglobulins diluted 1:500(Dako) or biotinylated rabbit anti-mouse immunoglobulins diluted 1:250 (Dako), respectively. The immunostaining was visualized by use of avidin, biotinylated horseradish peroxidase (Dako), and 3,3′-diaminobenzidine tetrahydrochloride. To visualize nuclei, sections were additionally stained by hematoxylin for 1 min.
The morphology of the material was analyzed by eosin/hematoxylin staining of adjacent sections. In control experiments performed to exclude nonspecific staining no or very faint diffuse intracellular staining of human fetal skeletal muscle cells was observed when the first antibody (anti-nestin) was replaced by preimmune serum from the same rabbit used for producing the anti-nestin antiserum.
RT-PCR of formalin-fixed paraffin-embedded tumor sections. Paraffin-embedded formalin-fixed tissues were cut into 10-μm thick sections and collected in Eppendorf tubes (7-8 sections/tube). The sections were deparaffinized in xylene twice for 30 min each, washed in ethanol, dried in vacuum, and incubated overnight at 37 °C with 100 μL of proteinase K(200 mg/mL) and 0.5 μL of RNasin (Promega). The material was thereafter incubated at 95 °C for 8 min to inactivate the protease. mRNA in the supernates was captured by use of Oligo(dT)25 Dynabeads (Dynal) and reversely transcribed by avian myeloblastosis virus-RT (Promega). Equal amounts of cDNA was used for PCR analysis of MyoD and GADPH expression. PCR was carried out for 27 cycles (95 °C for 30 s, 55 °C for 30 s, and 72°C for 90 s). PCR products were size-fractionated in 1% SeaKem agarose minigels in Trisborate-EDTA buffer, transferred to nylon membranes (Hybond-N, Amersham), and hybridized with probes specific for MyoD and GADPH.
PCR primers used for amplification of MyoD sequences (expected size, 466 bp) were: upstream 5′-CTC CTG AAA CCC GAA GAG CAC-3′, and downstream 5′-T CCA TCA TGC CGT CGT CGG AGC AGT T-3′, and for GADPH sequences (expected size 683 bp): upstream 5′-GTG AAG GTC GGA GTC AAC GGA TTT GG-3′, and downstream 5′-AT GCC AGT GAG CTT CCC GTT CAG CT-3′. Endlabeled oligonucleotides used as probes were MyoD: 5′-AAT ATC CAC TTT ACC AGA GTT AAA AGC AGC CCT GGT GAC CAG GCG CCC AA-3′, and GADPH: 5′-ATC ATG CCG TCG GAG CAG TTG GAG C-3′.
RESULTS
Immunohistochemical analysis of intermediate filament proteins in prenatal and adult human skeletal muscle. To learn whether the experimental conditions used for analysis of the tumor material allowed detection of nestin immunoreactivity in immature muscle, paraffin embedded fetal human muscle was analyzed for nestin expression. Using the anti-nestin antiserum no. 130, nestin immunoreactivity was clearly evident in skeletal muscle in a leg from a 10-wk human fetus, whereas in surrounding mesenchymal tissue it was not found (Fig. 1). Desmin immunoreactivity was also present in the muscle staining for nestin (not shown). In the same embryo, high levels of nestin were detected also in the spinal cord. At this developmental age, the skeletal muscle consists of centronucleated myotubes without cross-striation. Normal adult human skeletal muscle was clearly stained for desmin, but not for nestin(8) (data not shown).
Nestin and desmin expression in RMSs. We next analyzed a panel of 42 different pediatric soft tissue tumor samples for expression of nestin and desmin. The results are summarized in Table 1. Twenty-nine of the tumors were classified as RMS (23 embryonal RMS including 5 pleomorphic, 1 dense, and 1 spindle cell subtypes, and 6 alveolar RMS), and two embryonal sarcomas were suspected to represent a primitive form of embryonal RMS. The other tumors, including small cell tumors of infancy sometimes difficult to differentiate from RMS, were 2 Ewing's sarcomas, 1 neuroblastoma, 1 non-Hodgkin lymphoma, 1 embryonal ovarian carcinoma, 1 peripheral PNET tumor, 1 lung metastasis from a small cell tumor of unknown origin, 1 fibrosarcoma, 1 ganglioneuroblastoma, 1 ektomesenchymoma, and 1 Wilms' tumor with myogenic differentiation including frequent cross-striation.
Nestin immunoreactivity was present in all of the 29 presumed, as well as in the 2 suspected, RMSs. All 29 typical RMSs were positive also for desmin.Figure 2 illustrates an embryonal RMS positive for both nestin and desmin (case 1). Cross-striation was seen in only five RMS, all of which represented embryonal RMS, and they all expressed both nestin and desmin immunoreactivity. The nestin immunoreactivity, similar to desmin, was z-disc associated in the cross striated cells (Fig. 2,c and d). Two embryonal sarcomas (tumors nos. 30 and 31) were positive for nestin, but negative for desmin immunoreactivity (Fig. 3). Two undifferentiated tumors were classified as possible RMS before the detailed analysis associated with the present study. One of these was an undifferentiated metastasis in the lung, originally believed to be derived from a prior RMS, but without trait as such (case 34). The other tumor was originally classified as a possible RMS, but was reclassified as a Ewing's sarcoma (case 35). Neither of these tumors stained for nestin, desmin, myoglobin or α-actin. One Wilms' tumor with myogenous differentiation was positive for nestin as well as for desmin. The other non-RMS tumors analyzed here did not stain for nestin or desmin.
Alveolar RMS (case 17) with positive immunoreactivity for nestin (a) and desmin (b). In an embryonal RMS (case 9) cross-striated rhabdomyoblasts showed a banded pattern in nestin(c) and desmin (d) staining. The immunostainings were visualized by use of avidin and biotinylated horseradish peroxidase and counterstained with hematoxylin. Original magnification of a and b, ×200; c and d, ×400.
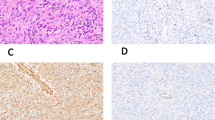
Embryonal sarcomas suspected to represent RMSs(a and b, case 30; c and d, case 31) stained with antibodies to nestin (a and c) and desmin(b and d). Note the absence of desmin staining in the tumor material, whereas nestin immunoreactivity is present. Immunostaining was visualized by avidin and biotinylated horseradish peroxidase and additionally stained with hematoxylin. Original magnification, ×250.
Nestin expression in tumor vessel endothelium and adjacent skeletal muscle. Cross-striated skeletal muscle of normal morphologic appearance in the vicinity of tumor cells was seen in several tumor samples. Strong nestin immunoreactivity was observed in the skeletal muscle surrounding seven RMSs and a peripheral PNET. All of these muscle fibers also stained with antibodies against desmin. Figure 4 shows strong z-disc associated nestin immunorectivity in muscle surrounding the peripheral PNET tumor. The nestin-positive muscle fibers had large, centrally located nuclei, indicating a regenerative origin. Nestin immunoreactivity was also seen in endothelial cells in 22 of the 42 analyzed tumor specimens. The endothelial nestin immunoreactivity was present in both RMS and in other small cell tumors.
RT-PCR analysis of MyoD expression. Two out of the 31 tumors diagnosed as typical or suspected RMS were positive for nestin, but did not stain with antibodies against desmin (tumor nos. 30 and 31). To further investigate the skeletal muscle origin of these two tumors, we analyzed the mRNA expression of the skeletal muscle-specific myogenic regulatory gene MyoD in these two and in two nonmuscle tumors represented by an embryonal carcinoma(case 41) and a Ewing's sarcoma (case 35) (Fig. 5). The two embryonal sarcomas diagnosed as possible RMS in one case represented a paratesticular undifferentiated mesenchymal tumor (case 30;Fig. 5,lane 4), and the other case an undifferentiated tumor located in the axilla (Case 31; Fig. 5,lane 1). Only the latter of these two possible RMS expressing nestin but not desmin contained MyoD transcripts at detectable levels (Fig. 5,lane 1). MyoD mRNA was not detected in the paratesticular mesenchymal tumor, indicating that it does not represent a RMS. (Fig. 5,lane 4). The two nonmuscle tumors did not express MyoD (Fig. 5,lanes 2 and 3). Transcripts for GADPH were detected in all tumor samples (Fig. 5).
RT-PCR of formalin-fixed paraffin-embedded tumor sections for detection of MyoD (left) and GADPH (right) mRNA. Lane 1, embryonal sarcoma suspected to represent a RMS (case 31); lane 2, embryonal carcinoma (case 41); lane 3, Ewing's sarcoma (case 35); lane 4, embryonal sarcoma suspected to represent a RMS (case 30). Expected sizes of MyoD and GADPH RT-PCR products are indicated in base pairs.
DISCUSSION
Nestin has recently been demonstrated to be a transient component of the dynamic intermediate filament network during muscle development(8, 13). The expression pattern of nestin during normal development suggests that skeletal muscle progenitor cells first express the intermediate filaments nestin and vimentin, later to be replaced by desmin. Desmin is a sensitive and clinically useful indicator of RMS(3, 4), but it is also found in many smooth muscle tumors(14, 15). The finding that nestin is expressed in immature skeletal muscle cells prompted us to compare the staining patterns of nestin and desmin in RMSs, and in some other small cell tumors sometimes difficult to distinguish from RMSs. We found that nestin immunoreactivity was present in all desmin expressing RMSs. Two additional undifferentiated small cell tumors suspected to represent primitive RMSs expressed nestin, but not desmin. One of these, a paratesticular tumor (case 30), is unlikely to represent a RMS since it did not contain any transcripts for the myogenic regulatory gene MyoD. This very poorly differentiated tumor was also negative for immunohistochemical staining for tissue plasminogen activator, episialin, S-100, leukocyte common antigen, α-fetoprotein, and human chorionic gonadotropin (data not shown). The other presumed RMS expressing nestin but not desmin (case 31), was positive for MyoD in RT-PCR analysis, and is considered to represent primitive RMS. The muscle-specific regulatory gene MyoD has been demonstrated to be a useful aid for demonstrating a skeletal muscle origin of tumors(5, 16, 17). The clinical use of MyoD analysis for RMS diagnosis is, however, presently limited due to lack of anti-MyoD antibodies useful for immunohistochemical detection on paraffin-embedded tissues. In nestin positive, desmin negative small cell tumors, further analysis of MyoD expression may be of value to determine whether the tumor contains myogenic components. One of the other types of small cell tumor analyzed, a Wilms' tumor with marked myogenic differentiation, was not surprisingly positive for nestin, as well as for desmin, whereas the other small cell malignant tumors were nestin-negative. Rhabdomyomatous differentiation in Wilms' tumors is a rather frequent phenomenon, associated with positive MyoD expression(17).
Our findings indicate that nestin is a useful complementary marker for RMS with high sensitivity but lower specificity, and that a minority of RMSs may express nestin but not desmin immunoreactivity. We think that the latter represent more primitive tumors than those expressing both nestin and desmin, in analogy with the switch in intermediate filament expression during normal myogenic differentiation(8).
Although we did not detect nestin immunoreactivity in 10 out of 11 small cell tumors, including Ewing's sarcoma, neuroblastoma, ganglioneuroblastoma, non-Hodgkin lymphoma, peripheral PNET, and an ovarian tumor, nestin has previously been demonstrated in tumors of neuroectodermal origin. Nestin is expressed in human CNS tumors, including central primitive neuroectodermal tumors, glioblastomas, astrocytomas, and oligodendrogliomas(11, 12). In this context it is interesting to note that nestin immunorectivity has been reported to be positive in cerebellar PNET oligodendrogliomas(11, 12), but was negative in the peripheral PNET (case 40). Rhabdomyoblastic differentiation is known in cerebellar PNET(18), whereas it has never been described in peripheral PNET. Nestin expression has also been documented in melanomas(19). All these reported cases of nestin-positive tumors, and the RMSs presented here, represent tumors of the central or peripheral nervous system, or skeletal muscle origin. It could be hypothesized that this tumor staining pattern reflects the expression pattern of nestin during normal embryonic development, with transient nestin expression in the developing central and peripheral nervous systems, and skeletal muscle(6, 8, 11, 12). This idea may be supported by the observation that immortalized cell lines from these tissues often express nestin(7, 20, 21). We speculate that the expression of nestin in immature cells of the skeletal muscle and nervous system, but not in the highly specialized muscle fibers, neurons, or glial cells reflects a change in requirements in intermediate filament polymerization or function during differentiation.
Somewhat surprisingly, in several cases we found nestin staining in skeletal muscle surrounding the tumor cells. Nestin is normally not expressed in mature muscle, but it is reexpressed in regenerating muscle(22). We believe that the positive nestin immunoreactivity in muscle cells in the vicinity of the tumor represents a paramalignant phenomenon. It may be related to induction of satellite cells to undergo regeneration. Alternatively nestin expression is reinduced in mature muscle fibers. However, the large, centrally located nuclei in the nestin-positive fibers indicate that they represent regenerating cells.
In summary, we have found nestin immunoreactivity in all analyzed typical RMSs, as well as in two very undifferentiated tumors regarded as embryonal sarcomas, one of which was diagnosed as a RMS due to expression of MyoD. Although nestin expression is not restricted to RMSs, it serves as a complement in the diagnosis of very undifferentiated, desminnegative RMSs.
Abbreviations
- RMS:
-
rhabdomyocarcoma
- IF:
-
intermediate filament
- RT:
-
reverse transcriptase
- GADPH:
-
glyceraldehyde-3-phosphate dehydrogenase
- PNET:
-
primitive neuroectodermal tumor
References
Pappo AS, Shapiro DN, Crist WM, Maurer HM 1995 Biology and therapy of pediatric rhabdomyosarcoma. J Clin Oncol 13: 2123–2139
Caillaud JM, Garard-Marchant R, Marsden HB, van Unnik AJ, Rodary C, Rey A, Flamant F 1989 Histopathological classification of childhood rhabdomyosarcoma: a report from the International Society of Pediatric Oncology Pathology panel. Med Pediatr Oncol 17: 391–400
Truong LD 1990 The diagnosis utility of desmin: a study of 584 cases and review of the literature. Am J Clin Pathol 93: 305–314
Parham DM, Webber B, Holt H, Williams WK, Maurer H 1991 Immunohistochemical study of childhood rhabdomyosarcomas and related neoplasms. Results of an intergroup rhabdomyosarcoma study project. Cancer 67: 3072–3080
Scrable HJ, Witte D, Shimada H, Seemayer T, Wang-Wuu S, Soukup S, Koufos A, Houghton P, Lampkin B, Cavenee W 1989 Molecular differential pathology of rhabdomyosarcoma. Genes Chromosom Cancer 1: 23–35
Lendahl U, Zimmerman LB, McKay RDG 1990 CNS stem cells express a new class of intermediate filament protein. Cell 60: 585–595
Fredriksen K, McKay RDK 1988 Proliferation and differentiation of the rat neuroepithelial precursor cells in vivo. J Neurosci 8: 1144–1151
Sejersen T, Lendahl U 1993 Transient Expression of the intermediate filament nestin during skeletal muscle development. J Cell Sci 106: 1291–1300
Sjöberg G, Jiang W-Q, Ringertz N, Lendahl U, Sejersen T 1994 Colocalization of nestin and vimentin/desmin in skeletal muscle cells demonstrated by three-dimensional fluorescence digital imaging microscopy. Exp Cell Res 214: 447–458
Donaldson SS, Draper GJ, Flamant F, Gerard-Marchant R, Mouriesse H, Newton WA, Lemerle J 1986 Topography of childhood tumors: pediatric coding system. Pediatr Hematol Oncol 3: 249–258
Dahlstrand J, Collins VP, Lendahl U 1992 Expression of the class VI intermediate filament nestin in human central nervous system tumors. Cancer Res 52: 5334–5241
Tohyama T, Lee VM-Y, Rorke LB, Marvin M, McKay RDG, Trojanowski JQ 1992 Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cells. Lab Invest 66: 303–313
Kachinsky A, Dominov J, Miller J 1994 Myogenesis and the intermediate filament nestin. Dev Biol 165: 216–228
Schurch W, Skalli O, Seemayer TA, Gabbiani G 1987 Intermediate filament proteins and actin isoforms as markers for soft tissue tumor differentiation. I. Smooth muscle tumors. Am J Pathol 128: 91–103
Rangdaeng S, Truong LD 1991 Comparative immunohistochemical staining for desmin and muscle-specific actin. A study of 576 cases. Am J Clin Pathol 96: 32–45
Hiti AN, Bogenmann E, Gonzales F, Jones P 1989 Expression of the MyoD1 muscle determination gene defines differentiation capability but not tumorigenicity of human rhabdomyosarcomas. Mol Cell Biol 9: 4722–4730
Dias P, Houghton PJ 1990 Myogenic regulatory protein(MyoD1) Expression in Childhood Solid Tumors: Diagnostic Utility in Rhabdomyosarcoma. Am J Pathol 137: 1283–1291
Kalimo H, Paljarvi L, Ekfors T, Pelliniemi J 1987 Pigmented primitive neuroectodermal tumor with multipotential differentiation in cerebellum (pigmented medullomyoblastoma). A case with light- and electron-microscopic, and immunohistochemical analysis. Pediatr Neurosci 13: 188–195
Florenes VA, Holm R, Myklebost O, Lendahl U, FodstadÖ 1994 Expression of the neuroectodermal intermediate filament nestin in human melanomas. Cancer Res 54: 1–3
Redies C, Lendahl U, McKay RDG 1991 Differentiation and heterogeneity in T-antigen immortalized precursor cell lines from mouse cerebellum. J Neurosci Res 30: 601–615
Renfranz PJ, Cunningham MG, McKay RD 1991 Region-specific differentiation of the hippocampal stem cell line HiB5 upon implantation into the developing mammalian brain. Cell 66: 713–29
Sjöberg G, Edström L, Lendahl U, Sejersen T 1994 Myofibers from Duchenne/Becker muscular dystrophy and myositis express the intermediate filament nestin. J Neuropathol Exp Neurol 53: 416–423
Acknowledgements
The authors thank Gabriella Dombos for excellent technical assistance, Dr. Niklas Pal for help in collecting tumor material, and Ulf Honberg for technical advice.
Author information
Authors and Affiliations
Additional information
Supported by grants from the Swedish Cancer Society (T.S., U.L.), the Swedish Child Cancer Fund (T.S., U.L.), the Swedish Institute (M.K), Stiftelsen Samariten (G.S.), and the Swedish Society for Medical Research(G.S).
Rights and permissions
About this article
Cite this article
Kobayashi, M., Sjöberg, G., Söderhäll, S. et al. Pediatric Rhabdomyosarcomas Express the Intermediate Filament Nestin. Pediatr Res 43, 386–392 (1998). https://doi.org/10.1203/00006450-199803000-00013
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199803000-00013
This article is cited by
-
Nestin expression in intact and hypertrophic myocardium of spontaneously hypertensive rats during aging
Journal of Muscle Research and Cell Motility (2024)
-
Nestin, a neuroectodermal stem cell marker, is expressed by bovine sertoli cells
Comparative Clinical Pathology (2012)
-
Nestin expression in astrocytic tumors delineates tumor infiltration
Brain Tumor Pathology (2010)
-
Nestin expression in osteosarcomas and derivation of nestin/CD133 positive osteosarcoma cell lines
BMC Cancer (2008)
-
Nestin expression in the cell lines derived from glioblastoma multiforme
BMC Cancer (2006)