Abstract
Primarily, our objectives were to compare system A amino acid transporter activity in the microvillous plasma membrane (MVM) of placentas from normally grown (appropriate for gestational age, AGA) and intrauterine growth-restricted (IUGR) fetuses delivered during the third trimester, as a whole and in relation to the severity of IUGR. Ten AGA and 16 IUGR pregnancies were studied at the time of elective cesarean section performed between 28 and 40 wk of gestation. Severity of IUGR pregnancies was assessed primarily by Doppler velocimetry and fetal heart rate monitoring. Placental MVM vesicles were prepared, and system A activity in these was measured. The transporter activity was significantly lower in IUGR compared with AGA pregnancies. Within the IUGR group system A activity was only significantly lower, compared with AGA, in cases that presented with a reduction in umbilical blood flow. We conclude that placental MVM system A activity is lower in IUGR compared with AGA pregnancies delivered during the third trimester. System A activity is related to the severity of IUGR.
Similar content being viewed by others
Main
Normal intrauterine growth relies on an adequate supply of nutrients from the mother to the fetus via the placenta. Recently, the availability of techniques to sample umbilical cord blood in utero(1, 2) has made it possible to measure plasma concentrations of various substrates and products of fetal metabolism under reasonably steady state conditions. Data obtained in this way have shown that the concentration of most amino acids in umbilical venous plasma from fetuses suffering from intrauterine growth restriction, during the third trimester, are lower than those in samples from appropriately grown for gestational age(AGA) fetuses(3, 4), whether or not the IUGR fetuses had normal oxygenation and acid-base balance(5). One explanation for these data is that there is a decrease in amino acid transport by the placenta of the IUGR fetus.
Amino acid transport by the placenta occurs predominantly via a transcellular mechanism utilizing specific transporter proteins in the microvillous (maternal facing) and basal (fetal facing) plasma membranes of the syncytiotrophoblast(6, 7). There are a variety of such transporters, selective for various classes of amino acids(6, 7). Direct evidence of a defect in placental amino acid transport was first obtained by Dicke and Henderson(8). These authors reported that uptake of AIB (a nonmetabolizable amino acid analog) by vesicles isolated from the MVM of the syncytiotrophoblast was significantly reduced in IUGR compared with AGA pregnancies. More recently, Mahendran et al.(9) showed that this was predominantly due to a reduction in the Vmax of the Na+-dependent system A amino acid transporter, which is specific for neutral amino acids with short side chains such as glycine and alanine. Both of these studies were conducted on placentas obtained from babies delivered at term and did not consider whether the activity of the transporter was related to the severity of the disease.
Therefore the primary aim of the present study was to determine whether:1) there is a lower activity of the system A transporter in MVM vesicles isolated from placentas of babies suffering from IUGR, delivered in the third trimester due to the severity of fetal compromise, compared with that in MVM vesicles from placentas of AGA babies; 2) the activity of the system A transporter is related to the severity of IUGR as assessed by FHR monitoring and umbilical arterial Doppler velocimetry; 3) there is any correlation between system A activity and biochemical indices of fetal well being.
There is evidence of an association, at term, between the activity of another transporter in the microvillous plasma membrane of the syncytiotrophoblast, the Na+/H+ exchanger, and birth weight(10). The function of this transporter in the placenta is not certain, but it does seem to have a role in the regulation of the intracellular pH of the syncytiotrophoblast(11). Therefore, particularly bearing in mind the tendency of the IUGR fetus to be acidemic(12), we took the opportunity afforded by this study to determine whether the activity of the Na+/H+ exchanger in the MVM of placentas from preterm IUGR fetuses is different from that of placentas from AGA fetuses.
METHODS
Pregnant patients were studied in the Department of Obstetrics and Gynecology of the San Paolo Institute of Biomedical Sciences. The protocol was approved by the San Paolo Institute Board. Informed consent was obtained from all pregnant women.
Clinical studies. Twenty-six patients having singleton pregnancies were studied at the time of elective cesarean section(i.e. without spontaneous or induced uterine contractions) performed between 28 and 40 wk of gestation after an overnight fast. Gestational age was calculated from the last menstrual period and confirmed by an ultrasonographic examination performed before 20 wk of gestation. Ten pregnancies terminated in the delivery of infants whose birth weights were AGA. Indications for cesarean section in the AGA pregnancies were repeat cesarean section (six cases), breech presentation (one case), maternal indications (three cases); none of the babies showed any signs of distress at delivery. Sixteen pregnancies were followed antenatally, and IUGR was diagnosed in utero by measurements of abdominal circumferences below the 10th percentile of reference values for fetuses of similar ages(13). All fetuses had normal karyotypes and no malformations at birth. All IUGR fetuses were electively delivered by cesarean section in the interest of the fetus. IUGR was confirmed at birth if the neonatal weight was below the 10th percentile according to Italian standards for birth weight and gestational age(14). None of these pregnancies was affected by preeclampsia or other maternal diseases.
In all cases general anesthesia was used for the cesarean section. Umbilical arterial and venous blood samples were withdrawn from a doubly clamped segment of the cord immediately after fetal extraction.
Biochemical analyses. Fetal blood samples were collected into heparinized 2.5-mL syringes that were immediately sealed and stored on ice. Hb concentration and oxygen saturation were measured in duplicate on a Radiometer(Copenhagen) OSM-3 oxymeter; oxygen content was calculated from the above variables according to the formula: pH, Po2, and Pco2 were measured on a Radiometer ABL 330 analyzer. The lactate concentration was measured in duplicate on a Yellow Springs Instrument Co.(Yellow Springs, Ohio) Analyzer (model 23L).
Severity of IUGR: velocimetry and heart rate measurement. As previously reported(5), IUGR fetuses were evaluated by assessment of Doppler velocimetry of the fetal umbilical artery and by FHR recordings performed immediately before cesarean section. Briefly, the wave form of the umbilical artery blood flow was measured with a coaxial pulsed Doppler velocimeter with a sample volume of 5 mm and high pass filters set at 100 Hz (Ultramark 5, APOGEE 800, ATL Seattle) and the PI was measured according to the simplified Gosling formula (systolic velocity minus diastolic velocity divided by mean velocity)(15). The criteria used to evaluate FHR tracings were the degree of variability, the presence of accelerations from the baseline, and the presence of decelerations in heart rate after Braxton-Hicks contractions. According to PI measurements and FHR patterns, IUGR fetuses were divided into the three subgroups we have previously described(5): group 1 (normal FHR and PI: 4 cases), group 2 (normal FHR, abnormal PI: 3 cases), and group 3 (abnormal FHR and PI: 9 cases).
Preparation of MVM vesicles. Immediately after delivery placentas were collected and weighed after trimming of the membranes. MVM were prepared from a portion of the placenta (approximately 100 g) using a homogenization and Mg2+ precipitation technique as previously described(16). Samples of the original homogenate and of vesicle suspensions were then stored at - 196 °C until further analysis.
Analysis of vesicle purity and measurement of system A and Na+/H+ exchanger activity. Homogenate samples and MVM vesicles were shipped in liquid nitrogen to the University of Manchester, where they were then stored at -20°C before further analysis. To determine the purity of the preparations, the enrichment of alkaline phosphatase activity in MVM vesicles compared with homogenate samples was measured as previously described(16). We excluded from further analysis any preparation in which the alkaline phosphatase enrichment was less than 10.
MeAIB is a nonmetabolizable amino acid analog that is specifically transported by system A(7). As system A is Na+-dependent, we measured its activity, at room temperature, as the difference in the initial rate of uptake by the vesicles of 14C-MeAIB in the presence and absence of an inward Na+ gradient exactly as previously described(9, 17). We have previously shown that uptake after 30 s provides a reasonable estimate of initial rate(9, 17), and this was confirmed during the course of this study (data not shown). In some experiments we also measured equilibrium uptake after incubation of the vesicles for 24 h(9, 17).
The Na+/H+ exchanger is blocked by amiloride at high concentrations(16). We therefore measured the activity of this transporter, also at room temperature, as the difference in the initial rate of uptake of 22Na by the vesicles in the presence and absence of 5 × 10-4 M amiloride as previously described(9, 16, 17). In these earlier studies we showed that uptake after 30 s approximates to initial rate and this was confirmed during the course of this study (data not shown). In some experiments we also measured equilibrium 22Na uptake after incubation of the vesicles for 2 h(9, 16, 17).
Statistical analysis. Data are presented as mean ± SEM. Statistical analysis was by t test, ANOVA followed by t test with the Bonferroni correction for number of groups, or, for data that was not normally distributed, the Mann-Whitney U test; a probability value of <0.05 was taken as significant. Associations between variables were analyzed by linear regression analysis using the least squares method.
RESULTS
The mean gestation at delivery of the AGA and IUGR fetuses was 36.4± 1.1 wk, n = 10 and 32.7 ± 0.8 wk, n = 16, respectively, and the difference was significant (p < 0.02;t test). As expected the mean birth weights of the two groups (2815± 223 g, n = 10, and 1333 ± 157 g, n = 16, for AGA and IUGR babies, respectively) was highly significantly different(p < 0.001; t test), as was placental weight (341± 22 g, n = 8 and 214 ± 23 g, n = 16;p < 0.01); see also Table 1.
Alkaline phosphatase enrichment of the MVM vesicles from the AGA group was 20.1 ± 1.6 (n = 10), not significantly different from that of the IUGR group (20.6 ± 1.3, n = 16). System A activity in the MVM vesicles from the placentas of the IUGR group (0.026 ± 0.004 nmol/mg protein/30 s, n = 16) was half (p < 0.001;t test) than in the vesicles from the placentas of the AGA group(0.053 ± 0.005 nmol/mg protein/30 s, n = 10). Equilibrium (24 h) uptake of 14C-MeAIB in the presence of Na+ was not different between the two groups (1.3 ± 0.7 nmol/mg protein, n = 8, and 3.1 ± 1.0 nmol/mg protein, n = 10, for AGA and IUGR groups, respectively), although the variation was quite wide.
The initial rate of Na+/H+ exchanger activity in the MVM vesicles from the two groups showed considerable variability and was generally lower than that which we have previously reported for unfrozen vesicles(9, 10, 16, 17). Even excluding one case in each group where the Na+/H+ exchanger activity was zero, the data were found not to be normally distributed. However, the median value for the activity of the exchanger in the vesicles from the AGA group (0.238 nmol/mg of protein/30 s, range 0.121-2.080; n = 8) was significantly(p < 0.02; Mann-Whitney U test) higher than that for the vesicles in the IUGR group (0.165 nmol/mg of protein/30 s, range 0.020-0.604; n = 10). Equilibrium (2 h) 22Na uptake in the absence of amiloride was not significantly different between the two groups(2.2 ± 0.3 nmol/mg of protein, n = 7, and 2.2 ± 0.4 nmol/mg of protein, n = 6, for AGA and IUGR groups, respectively).
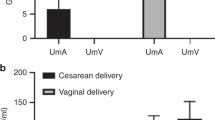
Table 1 presents data for the AGA and IUGR fetuses with the latter subdivided according to severity group. IUGR fetuses of group 3 underwent elective cesarean section at a significantly lower gestational age than AGA fetuses. Umbilical vein O2 content was significantly lower and lactate concentration significantly higher in group 3 compared with the AGA group, whereas umbilical vein Pco2, pH, and base deficit were not different between the groups. Figure 1 shows system A activity in the MVM vesicles from the AGA group and the subdivided IUGR group. As can be seen, system A activity in groups 2 and 3, but not group 1, was significantly lower than that in the AGA group.
Pooling data from both AGA and IUGR groups, there was a significant correlation between system A activity and gestational age (r2= 0.39, p < 0.001, n = 26). However, as shown in Figure 2, in AGA pregnancies with gestational ages ranging between 28 and 39 wk, no significant relationship was observed between system A activity and gestational age. By contrast, over a similar gestational age range, a significant relationship was observed between system A activity and gestational age in IUGR pregnancies (r2 = 0.3;p < 0.05; n = 16). Data from the IUGR pregnancies are presented with different symbols according to severity groups in Figure 2 and it is evident that the IUGR babies with the lowest gestational ages belong to groups 2 and 3, the most severely affected according to biophysical and biochemical parameters. There was no significant correlation between Na+/H+ exchanger activity and gestation.
Placental MVM system A activity in placentas from pregnancies with AGA fetuses (○) and with IUGR fetuses of group 1(•), group 2 (▪), and group 3 (▴) in relation to gestational age. Analyzing the data from all groups (AGA and IUGR) together there is a significant linear correlation (r2 = 0.39; p < 0.001). For AGA pregnancies alone the correlation coefficient for the linear regression is not significant (r2 = 0.16; p = 0.26). For IUGR pregnancies alone, the correlation is significant(r2 = 0.27; p < 0.05) as shown.
We investigated further the relationship between system A activity and umbilical vein O2 content, Po2, Pco2, pH, lactate concentration, and base deficit. Analysis of the data from IUGR and normal pregnancies together revealed a significant positive linear correlation between umbilical vein O2 content (r2 = 0.22,p < 0.02, n = 25), pH (r2 = 0.24,p < 0.02, n = 26), Po2 (r2 = 0.21; p < 0.02; n = 26) and system A activity and a significant negative linear correlation between umbilical vein lactate concentration (r2 = 0.29, p < 0.01, n= 24), base deficit (r2 = 0.28, p < 0.01,n = 26),), but not Pco2 (r2 = 0.01,n = 26) and system A activity. With regard to umbilical artery samples, there were significant negative linear correlations between system A activity and lactate concentration (r2 = 0.21, p< 0.05, n = 24) and base deficit (r2 = 0.3,p < 0.01, n = 24). In the AGA group, analyzed separately, a significant positive linear correlation was found only between umbilical arterial O2 content (r2 = 0.54, p< 0.05, n = 9) and Po2 (R2 = 0.48, p < 0.05, n = 9) versus system A activity. There were no significant relationships in the IUGR group analyzed separately.
DISCUSSION
The data reported here show that the activity of the system A amino acid transporter in the MVM of the syncytiotrophoblast is lower in placentas from fetuses suffering from IUGR and delivered during the third trimester due to the severity of compromise, compared with that in placentas from similarly delivered AGA fetuses. The present results also suggest that there is a relationship between system A activity and the degree of severity of compromise due to IUGR. There was no significant difference in alkaline phosphatase enrichment between the two groups of MVM vesicles, suggesting that, as at term(9), there was no difference in the purity of the preparations used. Furthermore, it should be noted that alkaline phosphatase enrichment and activity (data not shown) were similar in these frozen vesicles to that previously found in fresh vesicles(9, 17).
Because of the nature of the study it was necessary to freeze the MVM vesicles for varying lengths of time. This is not ideal as there is a suggestion from one study that freezing can affect the activity of amino acid transporters(18), although others did not find such an effect(19). However, the initial rate of system A activity that we measured in this study is well within the range we have reported previously for measurements on unfrozen vesicles from term placentas(9, 17). The equilibrium 14C-MeAIB uptake here is somewhat higher than that reported previously(9, 17), although the variability is also more marked, perhaps suggesting that previously frozen vesicles are less stable over several hours of incubation at room temperature than are fresh vesicles. However, it should also be noted that the vesicles from the two groups of placentas were treated identically and were collected concurrently, so that the storage conditions were unlikely to have confounded the data which we obtained.
Another possible confounding factor was that of gestation. To match our IUGR group we had to collect placentas from AGA fetuses, delivered preterm by cesarean section, in which there was no disease. Even though there is a paucity of such deliveries we were able to obtain placentas from AGA fetuses with a range of gestational ages similar to that of the IUGR group. However, it should be stressed that only 4 out of 10 cases were delivered before 36 wk in the control group compared with 13 of 16 in the IUGR group. We show that in this control group, although the mean gestation was slightly but significantly higher than the IUGR group, the activity of the system A transporter was unrelated to gestation. Consistent with this observation is a previous report that umbilical vein concentrations of amino acids do not change over the third trimester in AGA pregnancies(3). This therefore suggests that the difference in system A activity between the two groups was not the result of gestational differences per se. On the other hand the correlation between system A activity and gestation within the IUGR group may be considered as one of the indicators that there is a relationship with the severity of the disease.
The classification of IUGR pregnancies into three groups, based upon clinical biophysical measurements, has been proposed previously(5). Some further validation of the usefulness of the divisions is provided in the current study confirming, from biochemical measurements on umbilical vein blood obtained at cesarean section, a progressive worsening of oxygenation and acid base balance from group 1 to group 3. Fetuses in groups 2 and 3 were delivered at the earliest gestations, because of fear of their demise and their placental MVM vesicles had the lowest system A activity. It is worth emphasizing that the measurements of system A activity were made in Manchester without any knowledge of which IUGR group the vesicles had come from. Finally the overall significant linear relationship between MVM vesicle system A activity and fetal plasma oxygenation, pH, base excess, and lactate concentration also supports our contention of a relationship with fetal well being.
The initial rate of activity of the MVM Na+/H+ exchanger was significantly lower in the vesicles from the placentas of IUGR babies compared with that in vesicles from placentas of AGA babies. However, some caution needs to be observed in interpreting these data, as the values obtained were somewhat lower than those we have measured previously in unfrozen vesicles from term placentas(9, 16, 17). This is unlikely to be an effect of gestation as there was no significant correlation between Na+/H+ exchanger activity and this variable. Interestingly, equilibrium Na+ uptake, attained after 2 h of incubation, was identical to that reported previously for unfrozen, term vesicles(9, 16, 17), providing support for the suggestion that the higher than normal equilibrium values found for14 C-MeAIB was the result of alteration with extended time of incubation rather than any difference in initial vesicle volumes.
Despite these concerns the data reported here for the Na+/H+ exchanger are consistent with the previous observation of a significant correlation between the activity of this transporter and birth weight(10), made when pooling a large number of measurements on MVM vesicles prepared from placentas of IUGR babies, AGA babies, and macrosomic babies born to diabetic women. Na+/H+ exchanger has a role in the maintenance of the intracellular pH of the syncytiotrophoblast(11), and this might therefore be compromised in the smaller fetus. Furthermore, as the placenta undoubtedly has a role in the acid/base balance of the fetus, a lower placental Na+/H+ exchanger activity might contribute to the tendency of the IUGR baby to be acidemic(12) (Table 1).
It is very difficult to separate cause from effect in studying IUGR, but the recent observation that umbilical vein blood amino acid concentrations are reduced in all three IUGR severity groups(20) might suggest that decreased transporter activity follows alterations in fetal metabolism. However, transplacental amino acid fluxes in vivo will be dependent on the activity of the array of transporters in the microvillous and basal plasma membranes of the syncytiotrophoblast, which will, in turn, be dependent on the number and affinity of such transporters and the electrochemical gradients driving them. This complex system needs to be borne in mind when considering the implications of in vitro data such as that reported here. Nevertheless, recent data, which has shown, using stable isotopes, that in vivo materno-fetal fluxes of leucine are considerably greater than those of glycine(21), is consistent with data from MVM vesicles that the activity of the system L amino acid transporter (for which leucine is a substrate) is much greater than that of the system A amino acid transporter (for which glycine is a substrate)(17) and does suggest that the microvillous plasma membrane might be rate limiting in vivo. This finding also explains possible differences between umbilical venous amino acid concentrations and transporter activity in the three IUGR groups. The nonessential amino acids, like glycine, which are transported by system A, can be newly produced within the fetoplacental unit, and thus, in vivo data must consider both placental transport and fetoplacental metabolism.
Irrespective of this, the increasing number of observations suggesting that uptake of system A substrates is lower into MVM vesicles from placentas of IUGR babies compared with that into MVM vesicles from placentas of AGA babies(8, 9, 22), the association with severity of fetal compromise in IUGR observed in this study, the lower concentration of amino acids in umbilical vein blood from IUGR compared with that from AGA babies(3, 4, 20), together with the observation of reduced amino acid transfer across placentas of animals with experimentally induced growth restriction(6, 23), all suggest that reduced amino acid transfer across the human placenta is a major contributor to in utero growth restriction. It is therefore essential that future studies be directed toward characterizing the various components of placental amino acid flux, mentioned above, in IUGR and AGA pregnancies using both in vitro and in vivo techniques.

Abbreviations
- AGA:
-
appropriate for gestational age
- IUGR:
-
intrauterine growth restricted
- AIB:
-
aminoisobutyric acid
- MVM:
-
microvillous plasma membrane
- MeAIB:
-
methylaminoisobutyric acid
- PI:
-
pulsatility index
- FHR:
-
fetal heart rate
References
Daffos F, Capella-Pavlowsky M, Forestier F 1985 Fetal blood sampling during pregnancy with use of a needle guided by ultrasound: a study of 606 consecutive cases. Am J Obstet Gynecol 153: 655–660.
Marconi AM, Cetin I, Buscaglia M, Pardi G 1992 Midgestation cord sampling: what have we learned. Placenta 13: 115–122.
Cetin I, Corbetta C, Sereni LP, Marconi AM, Bozzetti P, Pardi G, Battaglia FC 1990 Umbilical amino acid concentrations in normal and growth-retarded fetuses sampled in utero by cordocentesis. Am J Obstet Gynecol 162: 253–261.
Economides DL, Nicolaides KH, Gahl WA, Bernardini I, Evans MI 1989 Plasma amino acids in appropriate and small-for-gestational-age fetuses. Am J Obstet Gynecol 161: 1219–1227.
Pardi G, Cetin I, Marconi AM, Lanfranchi A, Bozzetti P, Ferrazzi E, Buscaglia M, Battaglia FC 1993 Diagnostic value of blood sampling in fetuses with growth retardation. N Engl J Med 328: 692–696.
Morris FH, Boyd RDH, Mahendran D 1994 Placental transport. In: Knobil E and Neill JD (eds) The Physiology of Reproduction, 2nd Ed. Raven Press, New York, pp 813–860.
Moe A 1995 Placental amino acid transport. Am J Physiol 268:C1321–C1331.
Dicke JM, Henderson GI 1988 Placental amino acid uptake in normal and complicated pregnancies. Am J Med Sci 295: 223–227.
Mahendran D, Donnai P, Glazier JD, D'Souza SW, Boyd RDH, Sibley CP 1993 Amino acid (system A) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatr Res 34: 661–665.
Sibley CP, D'Souza SW, Doughty I, Boyd RDH, Glazier JD, Kuruvilla A, Mahendran D 1995 Birthweight and sodium/proton exchanger activity in the term human placenta. Placenta 16: 469–470.
Powell TL, Illsley NP 1996 A novel technique for studying cellular function in human placenta: gestational changes in intracellular pH regulation. Placenta 17: 661–668.
Soothill PW, Ajayi RA, Nicolaides KN 1992 Fetal biochemistry in growth retardation. Early Hum Dev 29: 91–97.
Todros T, Ferrazzi E, Groli C, Nicolini U, Parodi L, Pavoni M, Zorzoli A, Zucca S 1987 Fitting growth curves to head and abdomen measurements of the fetus: a multicentric study. J Clin Ultrasound 15: 95–105.
Parazzini F, Cortinovis I, Bortolus R, Fedele L 1991 Standard di peso alla nascita in Italia. Ann Ost Gin Med Perin 112: 203–246.
Gosling RG, King DH 1974 Continuous wave ultrasound as an alternative and complement to x-rays in vascular examinations. In: Reneman RS (eds) Cardiovascular Applications of Ultrasound. North Holland, Amsterdam, pp 266–282.
Glazier JD, Jones CJP, Sibley CP 1988 Purification and Na+ uptake by human placental microvillous membrane vesicles prepared by three different methods. Biochim Biophys Acta 945: 127–134.
Kuruvilla AJ, D'Souza SW, Glazier JD, Mahendran D, Maresh M, Sibley CP 1994 Altered activity of the System A amino acid transporter in microvillous membrane vesicles from placentas of macrosomic babies born to diabetic women. J Clin Invest 94: 689–695.
Karl PI, Teichberg S, Fisher SE 1991 Na+-dependent amino acid uptake by human placental microvillous membrane vesicles: importance of storage conditions and preservation of cytoskeletal elements. Placenta 12: 239–250.
Kudo Y, Yamada K, Fujiwara A, Kawasaki T 1987 Characterisation of amino acid transport systems in human placental brush-border membrane vesicles. Biochim Biophys Acta 904: 309–318.
Cetin I, Ronzoni S, Marconi AM, Perugino G, Corbetta C, Battaglia FC, Pardi G 1996 Maternal concentrations and fetal-maternal concentration differences of plasma amino acids in normal (AGA) and intrauterine growth restricted pregnancies. Am J Obstet Gynecol 174: 1575–1583.
Cetin I, Marconi AM, Baggiani AM, Buscaglia M, Pardi G, Fennessey PV, Battaglia FC 1995 In vivo placental transport of glycine and leucine in human pregnancies. Pediatr Res 37: 571–575.
Dicke JM, Verges DK 1994 Neutral amino acid uptake by microvillous and basal plasma membrane vesicles from appropriate and small for gestational age human pregnancies. J Matern Fetal Med 3: 246–250.
Ross JC, Fennessey PV, Wilkening RB, Battaglia FC, Meschia G 1996 Placental transport and fetal utilization of leucine in a model of fetal growth retardation. Am J Physiol 270: E491–E503.
Acknowledgements
The authors thank Dr. S. W. D'Souza, Dr. A. Kuruvilla, and Prof. R. D. H. Boyd for their contributions to this study. We gratefully acknowledge the contribution of Dr. K. Thornburg (Oregon Health Sciences University) to the start of the collaborative project and to the development of the studies. We also thank Dr. Anna Maria Baggiani for her work in the laboratory.
Author information
Authors and Affiliations
Additional information
Supported by Italian National Research Council (CNR)-Targeted Project“Prevention and Control Disease Factors”; Subproject“FATMA” no. 95.00880.PF41.115.21807; by European Community Biomed 1, Contract no. BMH1-CT94-1715; by a Medical Research Council grant (C.P.S.); and an Action Research Endowment Fund (Department of Child Health, University of Manchester).
Rights and permissions
About this article
Cite this article
Glazier, J., Cetin, I., Perugino, G. et al. Association between the Activity of the System A Amino Acid Transporter in the Microvillous Plasma Membrane of the Human Placenta and Severity of Fetal Compromise in Intrauterine Growth Restriction. Pediatr Res 42, 514–519 (1997). https://doi.org/10.1203/00006450-199710000-00016
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199710000-00016
This article is cited by
-
CHOP upregulation and dysregulation of the mature form of the SNAT2 amino acid transporter in the placentas from small for gestational age newborns
Cell Communication and Signaling (2023)
-
Fetal Sex Does Not Impact Placental Blood Flow or Placental Amino Acid Transfer in Late Gestation Pregnant Sheep With or Without Placental Insufficiency
Reproductive Sciences (2022)
-
Human placental uptake of glutamine and glutamate is reduced in fetal growth restriction
Scientific Reports (2020)
-
Reconciling the distinct roles of angiogenic/anti-angiogenic factors in the placenta and maternal circulation of normal and pathological pregnancies
Angiogenesis (2020)
-
Vasoactive Intestinal Peptide induces glucose and neutral amino acid uptake through mTOR signalling in human cytotrophoblast cells
Scientific Reports (2019)