Abstract
Prostaglandin E2 (PGE2) is an activator of bone remodeling, and increase levels of PGE2 are found in several disorders characterized by chronic inflammation. Bisphosphonates are used in the treatment of osteogenesis imperfecta (OI), an inherited disorder characterized by bone fragility and low bone mass. We evaluated the serum PGE2 (ng/mL) level in 16 children affected by OI (11 with mild and 5 with severe forms) at basal time and during treatment with neridronate. The levels of PGE2 in mild and severe forms were increased at basal time compared with controls (13.14 ± 4.2 versus 0.72 ± 0.05, p < 0.01; 15.1 ± 1.5 versus 0.72 ± 0.05, p < 0.01, respectively) and showed a significant decrease after the second (T1) cycle of treatment (mild: 4.97 ± 5.0 versus 13.14 ± 4.2, p < 0.01; severe: 5.32 ± 4.5 versus 15.1 ± 1.5, p < 0.01) with a further significant decrease after the fourth (T2) cycle. The high basal PGE2 levels in OI, a noninflammatory disorder, could be explained by stress-induced release mediated by inducible cyclooxygenase-2-catalyzed pathway. The reduction obtained by treatment with bisphosphonates could be attributed to a direct pharmacological effect since these drugs has been reported to modulate the release of proinflammatory mediators.
Similar content being viewed by others
Main
Several in vitro and in vivo studies have evaluated the effect of various cytokines on the differentiation and metabolism of bone cells. Osteoblast can regulate the secretion of cytokines that are produced in the bone microenvironment and influence its remodeling (1).
Prostaglandins, especially prostaglandin E2 (PGE2), are known to be potent activators of bone remodeling and have been reported as having both anabolic and catabolic effect on bone (2). Furthermore, PGE2 can modulate type I and type III collagen production with preferential loss of type I collagen in normal and in mutant fibroblasts (3). In the release of prostaglandins, at least two types of enzymes are involved: the cyclooxygenase-1 (COX-1), present as constitutively express isoform and the COX-2, generally considered as inducible isoform. The COX-2 is a ubiquitous enzyme responsible for the release of high amounts of prostaglandins in several pathologic events. COX-2 and PGE2 are involved in both cytokine-mediated osteoclast activation and osteoblast bone formation (4).
Osteogenesis imperfecta (OI) is an inherited disorder characterized by increased bone fragility and low bone mass (5). Four types are commonly distinguished on the basis of clinical and genetic features although overlaps are often observed (6). In most OI patients, the disease is caused by mutations in the collagen type I gene. The main pathophysiological effect is the production of an abnormal matrix that does not respond to mechanical loads. In compensation, the osteoblast population increases and osteoblast activity is raised, leading to a high bone turnover rate. The increased bone turnover explains the bone loss characterizing the clinical picture of the disease (7).
Bisphosphonates (bb) are currently used in the treatment of several forms of the disease. The main effect of the pharmacologically active bb is the reduction of bone turnover through decreasing bone reabsorption (8). In addition, it has been recently reported that bb modulate the release of proinflammatory mediators (9,10). Neridronate is an amino-bisphosphonate structurally similar to alendronate and pamidronate and was recently registered in Italy for the treatment of OI (11).
This led us to design a study to evaluate the serum level of PGE2 in children affected by OI at basal time and during treatment with neridronate.
MATERIALS AND METHODS
Subjects.
Sixteen prepubertal children affected by OI, 8 males and 8 females, aged from 2 to 11 y (median 6.9), outpatients in the Department of Pediatrics of University of Roma “La Sapienza,” were enrolled in the study. Patients were diagnosed on the basis of clinical and radiologic features according to the Sillence classification (6) and included 11 children with mild form (type I) and 5 with severe forms of OI (type III/IV) (Table 1). The patients were not receiving medication and were on a free diet with normal dietary protein and calcium intake. None of the patients was previously treated with bb, and routine hematological parameters, including inflammatory markers, were in the normal range. This study does not include patients who had experienced fractures, including vertebrae, in the previous 6 mo.
The treatment was started with cyclical neridronate infusion every 3 mo. Each cycle consisted of one infusion at a dose of 2 mg/kg/body weight diluted in 250 mL of saline solution. No adverse side effects were noted apart from the well-known acute phase reaction within 24–48 h after first infusion cycle. Levels of serum PGE2 and biochemical parameters of bone metabolism (serum calcium, inorganic phosphorus, creatinine, total and bone alkaline phosphatase), and urinary creatinine, calcium, and c-terminal telopeptide of collagen-I (CTx) were assayed at baseline (T0) and immediately before the third (T1) and fifth (T2) cycle of treatment (6 and 12 mo after the start of treatment, respectively). Patients were fasting at the time of blood and urine sampling. Urinary assays were performed using the second void sample of the morning.
Bone mineral density (BMD) at lumbar spine (L1–L4) was measured by dual-energy x-ray absorptiometry (QDR 4500 A; Hologic, Waltham, MA) at baseline (T0) and after 6 mo (T1) and 12 mo (T2) of treatment.
The reference normal control group consisted of 16 healthy children matched for sex and age (8 males, 8 females; age: 4–12 y, median 7.4 y) who underwent routine hematological assessment. Control values have been used to compare with pretreatment (T0) values of patients.
Informed consent was obtained from the parents of each patient before the start of treatment. The study was approved by the Ethics Committee of the Department of Pediatrics of University “La Sapienza” Roma.
PGE2 serum level determination.
Serum samples were obtained using a serum separator tube test (SST) allowing samples to clot for 30 min before centrifugation at approximately 1000 × g. When samples were not tested immediately, a prostaglandin synthetase inhibitor, such as indomethacin, was added at approximately 10 μg/mL final concentration before storage at –20°C.
PGE2 concentrations were determined by enzyme immunoassay, using a high-sensitivity ELISA kit (R & D Systems, Minneapolis, MN). All serum samples required an appropriate dilution (10–100 fold) depending on the amount of PGE2 detectable. Assays were performed according to the manufacturer's instructions. The PGE2 standard curve in assays carried out with the high sensitivity option ranged from 0.0196 ng/mL to 1.250 ng/mL, whereas with the regular sensitivity option ranged from 0.039 ng/mL to 2.5 ng/mL. The intra- and interassay coefficients of variation were below 10% and 12%, respectively. The minimum detectable dose (MDD) was 0.025 ng/mL.
Statistical analysis.
Data are reported as mean ± SD. The data do not appear to be normally distributed and may be log normal. In any case, the variance of the values of PGE2 in the subjects with OI is very much greater than that of the controls. We have therefore applied the Mann-Whitney nonparametric test to compare the OI subjects with the controls and the Wilcoxon test to compare the values of PGE2, BMD, and biochemical parameters of bone metabolism between T0, T1, and T2. Correlations were performed by Spearman's rank correlation test. A result is considered statistically significant if p < 0.05.
RESULTS
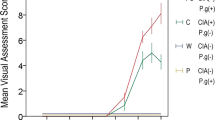
The basal level of serum PGE2 (ng/mL) of 11 patients affected by mild OI (type I) and the 5 patients affected by severe OI (type III/IV) showed values significantly higher in respect to the controls (mean ± SD: 13.14 ± 4.2 versus 0.72 ± 0.05, p < 0.01; and 15.1 ± 1.5 versus 0.72 ± 0.05, p < 0.01, respectively) (Fig. 1). No significant correlations were found between basal serum PGE2 levels, BMD, and biochemical parameters of bone metabolism, including bone apposition (bone alkaline phosphatase) and reabsorption (urinary CTx/creatinine) markers.
Individual and mean serum concentration (ng/mL) of PGE2 in 11 children affected by mild (•) and 5 children affected by severe (▴) OI in treatment with cyclical neridronate infusion every 3 mo. The analysis was performed at baseline (TO) and after the second (T1) and fourth (T2) cycle of treatment. The horizontal solid and dashed lines represent, respectively, mean ± 2 SD of control values. Basal levels (T0) of PGE2 are highly increased in respect to the control values (p < 0.01; Mann-Whitney test) and have been progressively reduced during neridronate treatment (T0 vs T1: p < 0.01; T1 vs T2: p < 0.01; Wilcoxon test). No differences were observed between mild and severe OI.
After two cycles of neridronate treatment (T1), a significant reduction of serum PGE2 in respect to the basal (T0) levels was observed both in mild and severe forms (mean ± SD: 4.97 ± 5.0 versus 13.14 ± 4.2; p < 0.01 and 5.32 ± 4.5 versus. 15.1 ± 1.5; p < 0.01, respectively). At this time, 3 out of the 11 patients with mild (27.3%) and 1 out of the 5 patients with severe form (20%) showed values within the normal range (Fig. 1). Following the fourth infusion (T2), there was a further significant reduction of serum PGE2 levels and 7 out of the 11 patients with mild (63.6%) and 3 out of the 5 patients with severe OI (60%) normalized their PGE2 levels (Fig. 1). There were no statistically significant differences in serum PGE2 levels between mild and severe forms at baseline and during bb treatment.
The values of BMD and biochemical parameters of bone metabolism are reported in Table 1. The percentage variation in respect to the baseline of urinary CTx/creatinine ratios and serum BMD during neridronate treatment are reported in Figure 2. Serum BMD showed a significant increase after the second cycle of treatment (T1) with a further significant increase after the forth cycle (T2) whereas urinary CTx/creatinine ratios showed a parallel decrease.
Comparison of urinary CTx / creatinine ratios (A) and serum BMD (B) in 11 children affected by mild (dashed lines) and 5 children affected by severe (solid lines) OI in treatment with cyclical neridronate infusion every 3 mo. The analysis was performed at baseline (TO) and after the second (T1) and fourth (T2) cycle of treatment. Values represent percentage variation in respect to the baseline. *Statistically significant in respect to the previous time (p < 0.05, Wilcoxon test).
No significant correlation was found between percentage variation (T0–T2) of serum PGE2 level and percentage variation (T0–T2) of BMD or biochemical bone parameters.
DISCUSSION
Our study provides evidence for two remarkable results. The first is that serum PGE2 basal levels are increased in children with OI, a disease characterized by high bone turnover rate, in which there is no evidence of chronic prophlogistic activation. Although extensively studied, the role of prostaglandins on bone metabolism is unclear and most likely bimodal. In fact, PGE2 acts as a potent stimulator of bone reabsorption in several disorders that are characterized by chronic inflammation, including osteoarthritis and periodontitis (12,13). Otherwise, a high basal plasma level of PGE2 positively correlates with bone formation markers in patients with ulcerative colitis, thus suggesting a protective role of PGE2 against bone loss in these patients (14). These results are an indication of the complex role of this cytokine in regulating bone remodeling.
An intriguing question is why PGE2 are increased in children affected by OI. Osteocytes are generally considered to be the bone mechanosensory cells that translate a mechanical signal into biochemical, bone metabolism-regulating stimuli necessary for the adaptive process. Prostaglandins are an important part of this mechano-biochemical signalling (15–17). Stress-induced PGE2 release has been well-investigated in vitro study, suggesting an activation mediated by the inducible COX-2-catalyzed pathway (18). It is well known that in OI, the genetic defect in the osteoblast interferes with the multiple mechanisms that normally ensure adaptation of the skeleton to the increasing mechanical need during growth (7). Our results suggest that in patients with OI the increased basal serum PGE2 levels can be attributed to excessive PGE2 release, perhaps mediated by COX-2 activation induced by mechanical stress in bone tissue.
Whether the role of PGE2 in the pathogenesis of OI has an importance comparable with that reported in inflammatory-mediated bone loss conditions is not known. We correlated basal serum PGE2 levels and BMD and biochemical parameters of bone remodeling, but no statistically significant results were found. These could be partly related to the small size of the study and the heterogeneity of the disease.
The second result of our study is the dramatic decrease of PGE2 levels after four cycles (1 y) of bb treatment. In vitro and in vivo studies have demonstrated that various bb modulate the release of proinflammatory mediators (9,10) and inhibit PGE2 synthesis induced by phlogistic or mechanical stimuli (13,18,19). These studies postulate that the inhibition of endogenous PGE2 production may be involved in the mechanism of action of bb (20). Recently, Liu et al. (18) found that clodronate inhibited the mechanical stress-induced production of PGE2, suggesting that inhibitory effect on osteoclast may be due, at least in part, to the inhibition of COX-2-dependent PGE2 production. Therefore, we hypothesize that even in OI the inducible form (COX-2) could be responsible for both high basal PGE2 release and response to bb administration.
In agreement with previous results (5,8), we observed a significant increase of BMD with a parallel decrease of urinary CTx/creatinine ratios during bb treatment (Fig. 2) but no significant correlation between PGE2 changes and variation of BMD or biochemical parameters of bone metabolism. The lack of a statistically significant effect on bone accrual, despite the significant reductions in the level of PGE2, may be explained by the occurrence of biochemical changes before clinically detectable effects take place. In any case, further studies are needed to better elucidate these points.
In conclusion, in the present study we observed very high basal serum PGE2 levels in children affected by OI that are lowered by neridronate treatment. These results could be important for the understanding of the role of metabolic factors in the pathogenesis of OI and the mechanism of action of bb. This may have important implications for the management of bone loss in noninflammatory diseases associated with prostaglandins activation.
Abbreviations
- bb:
-
bisphosphonates
- BMD:
-
bone mineral density
- COX-2:
-
cyclooxygenase-2
- CTx:
-
C-terminal telopeptide of collagen-I
- OI:
-
osteogenesis imperfecta
- PGE2:
-
prostaglandin E2
References
Heymann D, Rousselle AV 2000 Gp130 cytokine family and bone cells. Cytokine 12: 1455–1468
Kawaguchi H, Pilbeam CC, Harrison JR, Raisz LG 1995 The role of prostaglandins in the regulation of bone metabolism. Clin Orthop Relat Res 313: 36–46
Steinmann BU, Abe S, Martin GR 1982 Modulation of type I and type III collagen production in normal and mutant human skin fibroblast by cell density, prostaglandin E2 and epidermal growth factor. Coll Relat Res 2: 185–195
Liu XH, Kirschenbaum A, Yao S, Levine AC 2005 Cross-talk between the interleukine-6 and prostaglandin E2 signaling system results in enhancement of osteoclastogenesis through effects on the osteoprotegerin\receptor activator on nuclear factor-κB(RANK) ligand\RANK system. Endocrinology 146: 1991–1998
Rauch F, Glorieux FH 2004 Osteogenesis imperfecta. Lancet 363: 1377–1385
Sillence DO, Senn A, Danks DM 1979 Genetic heterogeneity in osteogenesis imperfecta. J Med Genet 16: 101–116
Rauch F, Travers R, Parfitt AM, Glorieaux FH 2000 Static and dynamic bone histomorphometry in children with osteogenesis imperfecta. Bone 26: 581–589
Glorieux FH 2007 Experience with bisphosphonates in osteogenesis imperfecta. Pediatrics 119: S163–S165
Dehghani F, Conrad A, Kohl A, Korf HW, Hailer NP 2004 Clodronate inhibits the secretion of proinflammatory cytokines and NO by isolated microglial cells in excitotoxically injured organotypic hippocampal slice cultures. Exp Neurol 189: 241–251
Tuominen OM, Ylitalo-Heikkala R, Vehmas TI, Mucha I, Ylitalo P, Riutta A 2006 Effect of bisphosphonates on prostaglandin E2 and thromboxane B2 production in human whole blood and monocytes stimulated by lipopolysaccharide and A23187. Methods Find Exp Clin Pharmacol 28: 361–367
Adami S, Gatti D, Colapietro F, Fracassi E, Braga V, Rossini M, Tatò L 2003 Intravenous neridronate in adults with osteogenesis imperfecta. J Bone Miner Res 18: 126–130
McCoy JM, Wicks JR, Audoly LP 2002 The role of prostaglandins E2 receptors in the pathogenesis of arthritis. J Clin Invest 110: 651–658
Buduneli E, Vardar S, Buduneli N, Berdeli AH, Turkoglu O, Baskesen A, Atilla G 2004 Effect of combined systemic administration of low-dose doxycycline and alendronate on endotoxin-induced periodontitis in rats. J Periodontol 75: 1516–1523
Wiercinska-Drapalo A, Jaroszewicz J, Tarasow E, Flisiak R, Prokopowicz D 2005 Transforming growth factor beta(1) and prostaglandin E2 concentrations are associated with bone formation markers in ulcerative colitis patient. Prostaglandins Other Lipid Mediat 78: 160–168
Ajubi NE, Klein-Nulend J, Alblas MJ, Burger EH, Nijweide PJ 1999 Signal transduction pathways involved in fluid flow-induced PGE2 production by cultured osteocytes. Am J Physiol 276: E171–E178
Burger EH, Klein-Nulend J 1999 Mechanotransduction in bone–role of the lacuno-canalicular network. FASEB J 13: S101–S112
Zhao W, Byrne MH, Wang Y, Krane SM 2000 Osteocyte and osteoblast apoptosis and excessive bone deposition accompany failure of collagenase cleavage of collagen. J Clin Invest 106: 941–949
Liu L, Hgarashi K, Haruyarma N, Sacki S, Shinoda H, Mitani H 2004 Effect of local administration of clodronate on orthodontic tooth movement and root resorption in rats. Eur J Orthod 26: 469–473
Igarashi K, Hirafuji M, Adachi H, Shinoda H, Miltami H 1997 Effect of bisphosphonates on alkaline phosphatase activity, mineralization, and prostaglandin E2 synthesis in the clonal osteoblast-like cell line MC3T3-E1. Prostaglandins Leukot Essent Fatty Acids 56: 121–125
Liu L, Igarashi K, Kanzaki H, Chiba M, Shinoda H, Mitani H 2006 Clodronate inhibits PGE2 production in compressed periodontal ligament cells. J Dent Res 85: 757–760
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was in part supported by grants from M.I.U.R. 2005–2006.
Rights and permissions
About this article
Cite this article
D'Eufemia, P., Finocchiaro, R., Celli, M. et al. High Levels of Serum Prostaglandin E2 in Children with Osteogenesis Imperfecta Are Reduced by Neridronate Treatment. Pediatr Res 63, 203–206 (2008). https://doi.org/10.1203/PDR.0b013e31815efd63
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e31815efd63
This article is cited by
-
Cyclic bisphosphonate therapy reduces pain and improves physical functioning in children with osteogenesis imperfecta
BMC Musculoskeletal Disorders (2018)
-
Association between spondylolisthesis and L5 fracture in patients with Osteogenesis Imperfecta
European Spine Journal (2017)
-
Impaired bone remodeling in children with osteogenesis imperfecta treated and untreated with bisphosphonates: the role of DKK1, RANKL, and TNF-α
Osteoporosis International (2016)