Abstract
The objective was to determine in infants with perinatal depression whether the relative concentrations of N-acetylaspartate and lactate in the neonatal period are associated with (1) neurodevelopmental outcome at 30 mo of age or (2) deterioration in outcome from age 12 to 30 mo; and to determine whether socioeconomic factors are associated with deterioration in outcome. Thirty-seven term neonates were prospectively studied with single-voxel proton magnetic resonance spectroscopy of the basal nuclei and intervascular boundary zones. Thirty-month outcomes were classified as normal [if Mental Development Index of the Bayley Scales of Infant Development (MDI) >85 and neuromotor scores (NMS) <3;n = 15], abnormal [if MDI ≤85 and/or NMS ≥3 at 12 and 30 mo;n = 11], or deteriorated [if normal at 12 mo and abnormal at 30 mo (MDI ≤85 or NMS ≥3);n = 11]. Thirty percent (11/37) of our cohort deteriorated between 12 and 30 mo. N-acetylaspartate/choline decreased across the groups ordered as normal, deteriorated, and abnormal [in basal nuclei (p ≤ 0.001) and intervascular boundary zones (p = 0.04)], but was not different between the normal and deteriorated groups (p = 0.08). Lactate/choline similarly increased across the groups [in basal nuclei (p = 0.01) and intervascular boundary zones (p = 0.05)]. The odds of deterioration, if normal at 12 mo, increased by a factor of 5.1 (95% confidence interval: 1.3–19.8) with each decrease in one of four household income strata. Infants with perinatal depression are at high risk of developmental deterioration between 12 and 30 mo of age, particularly if in a lower income home or with intermediate values of cerebral metabolites on neonatal proton magnetic resonance spectroscopy.
Similar content being viewed by others
Main
A number of studies have demonstrated that 1H-MRS can be used to predict neurodevelopmental outcome at 12 mo in term-born infants with perinatal depression (1–3). No study to date, however, has associated relative concentrations of NAA or lactate, as measured by 1H-MRS, with longer-term neurodevelopmental outcome. The purpose of this study was to determine, in infants with perinatal depression, (1) whether 1H-MRS results are associated with neurodevelopmental outcome at 30 mo of age, and (2) whether the relative concentrations of NAA and lactate in the neonatal period are associated with changes in neurodevelopmental status from age 12 mo to 30 mo. Given that socioeconomic factors impact long-term developmental outcome of ICN graduates (4, 5), we sought to determine the contribution of socioeconomic factors to changes in outcome at 30 mo of age in this cohort.
METHODS
We assembled a prospective cohort of term neonates with perinatal depression to study the MR predictors of neurodevelopmental outcome. This cohort, extensively described elsewhere (2), was derived from screening 5389 consecutive term neonates born in or transferred to our institution's ICN since 1994. The cohort comprises 126 infants studied with MR imaging (MRI) with one of the following indicators of perinatal depression: (1) umbilical artery pH <7.1; (2) umbilical artery base deficit >10; or (3) 5-min Apgar score ≤5. Newborns were excluded if (1) gestational age was <36 wk, or (2) there were suspected or confirmed congenital malformations, congenital metabolic disease, or congenital infections. The protocol was approved by the Committee on Human Research at the University of California San Francisco. Infants were studied only after voluntary informed consent was obtained from their parents.
Since the introduction of 1H-MRS as part of the study protocol in 1995, we have studied 91 newborns. Five of these 91 newborns have expired and 14 have been lost to follow-up. Of those studied with 1H-MRS, 61 have completed their 12-mo follow-up and 37 of these newborns have also completed their 30-mo follow-up. This consecutive series of 37 patients who completed both the 12- and 30-mo follow-up assessments comprises the group reported in this article.
MR data.
MRI and spectroscopy were done at a median of 6 d of life (range 2–13). The same MR scanner and techniques were used for the entire cohort. The newborns were imaged as soon as they were stable enough to be transported safely to the MR scanner and imaging time was available. MRI of the brain in all newborns included 4-mm (1-mm “gap”) sagittal spin-echo (SE) [500/11/2 (TR/TE/excitations)], 4-mm (1-mm “gap”) axial SE (500/11/2) images, and 4-mm (2-mm “gap”) axial SE (3000/60,120/1) images through the entire brain. MR abnormalities in the BN and frontal IBZ were scored using a system previously validated for the determination of neuromotor outcome (6). This system scores both regions on the T1 sequence and the first echo of the T2 sequence separately. Using this system, MRI abnormalities were scored in the BN as follows: 0 = normal or isolated focal cortical infarct; 1 = abnormal signal in thalamus; 2 = abnormal signal in thalamus and lentiform nucleus; 3 = abnormal signal in thalamus, lentiform nucleus, and perirolandic cortex; and 4 = more extensive involvement. Findings in the IBZ were scored as follows: 0 = normal; 1 = single focal infarction; 2 = abnormal signal in anterior or posterior watershed white matter; 3 = abnormal signal in anterior or posterior watershed cortex and white matter; 4 = abnormal signal in both anterior and posterior watershed zones; and 5 = more extensive cortical involvement.
Proton spectra were acquired from two separate 5-cm3 voxels, one centered on the BN, including the lentiform nucleus and thalamus (37 newborns), and the other from the frontal IBZ (36 newborns) (Fig. 1) (2). 1H-MRS was performed using the point-resolved spectroscopy (PRESS) technique to acquire long echo-time spectra from approximately 5.5 cm3 of tissue for both regions. The spectrum for each location was acquired using the PROBE (PROton Brain Exam, General Electric, Milwaukee, WI, U.S.A.) sequence in under 5 min with a TR of 2 s, a TE of 288 ms, and a total of 128 acquisitions. The 1H-MRS timings and voxel localization were chosen to maximize the detection of lactate and NAA, while minimizing extracranial adipose tissue contamination of the spectra (2). The voxels used were the same in all subjects, with the hemisphere for the voxels chosen to best fit with the imaging planes. After acquisition, the 1H-MRS data were transferred off-line and analyzed on a SPARC Workstation (Sun Microsystems, Mountain View, CA, U.S.A.) using spectral quantitation software developed in our laboratory (2). The 1H-MRS data were Fourier transformed and baseline fitted. The peak areas were integrated for the choline (trimethylamine), creatine, NAA, and lactate resonances.
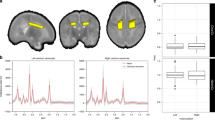
(A) Location of 1H-MRS voxels demonstrated on axial T2 images. The BN voxel includes lentiform nuclei, ventrolateral thalamus, and posterior limb of the internal capsule. The IBZ voxel includes primarily white matter in the region between the anterior cerebral artery and middle cerebral artery distributions. (B) Representative proton spectra of a newborn with normal neurodevelopmental outcome (voxels as shown, PRESS technique with TR 2 s, TE 288 ms, and 128 acquisitions). Choline (Cho), creatine (Cr), and NAA peaks are labeled. Note that the amplitude of the NAA peak is intermediate between that of the choline and creatine peaks. Note also the absence of a lactate peak. (C) Representative proton spectra of a newborn with abnormal neurodevelopmental outcome (voxels as shown, PRESS technique with TR 2 s, TE 288 ms, and 128 acquisitions). Note the presence of a lactate doublet and relative diminution of NAA compared with the spectrum in B.
Neonatal condition.
To quantify and summarize the clinical condition of our cohort in the neonatal period, 32 of the newborns were prospectively scored using the Score for Neonatal Acute Physiology–Perinatal Extension (SNAP-PE). SNAP-PE scores the worst physiologic derangement in each organ system during the first 24 h after admission, with increasing scores reflecting more severe derangement. This validated measure of illness severity is important for the prediction of neonatal mortality and was used here as an estimate of the overall clinical condition of the newborns (7, 8).
Developmental examinations.
All patients were examined at 12 and 30 mo of age. At both assessments, cognitive development was assessed by administering the MDI of the Bayley Scales of Infant Development II (BSID II) (9). At each assessment, a pediatric neurologist blinded to the neonatal course and imaging results of the infant performed a standardized neurologic exam. The neurologist scored neuromotor outcome using a validated NMS: 0 = normal; 1 = abnormal tone or reflexes or primitive reflexes; 2 = abnormal tone and reflexes; 3 = decreased power and tone or reflex abnormality; 4 = cranial nerve involvement and any motor abnormality; and 5 = cranial nerve involvement and spastic quadriparesis (10).
Outcome classification.
Infants were classified a priori as functionally normal, if the MDI was in the normal range (>85) and the NMS was <3 (no abnormality of motor power) at 30 mo; as abnormal, if MDI ≤85 (borderline or below) and/or NMS ≥3 (abnormality of motor power) at 30 mo; or as deteriorated, if normal (MDI >85, NMS <3) at 12 mo and abnormal (MDI ≤85, NMS ≥3) at 30 mo. The cutoff values for the NMS and the MDI were chosen to identify those children who would be targeted for rehabilitation or special educational services.
Socioeconomic condition.
At the 30-mo assessment, the primary caregiver's socioeconomic condition was assessed using the four-factor index of social status (Hollingshead A, unpublished working paper, Yale University, New Haven, CT, 1975). Total annual household income was stratified as (1) ≤$15,000; 2) $15,000–$35,000; (3) $35,000–$75,000; and (4) ≥$75,000. Maternal education was stratified as (1) did not complete high school; (2) completed high school (grade 12); (3) at least one year of college; (4) completed college; and (5) graduate school. Caregivers were also asked to identify whether their child was receiving any rehabilitation service (e.g. physical therapy, occupational therapy, speech therapy, developmental program).
Total annual household income was provided in 30 of 37 cases; nonresponders were distributed evenly among the groups (three normal, two deteriorated, and two abnormal). Twenty-four caretakers provided information on the highest level of maternal education achieved. Of the nonresponders, three were normal, six deteriorated, and four were abnormal. In the 21 cases where both estimates were provided, household income and maternal education were highly correlated (Spearman's ρ = 0.73, p = 0.0002). Given this high correlation among the two variables and the poor response rate regarding maternal education, only total annual household income was used in the analysis as a measure of the infant's socioeconomic condition.
Data analysis.
Statistical analysis was performed using Stata (Stata Corporation, College Station, TX, U.S.A.). Univariate comparisons of the MDI, NMS, and MRI scores of the outcome groups were made with t tests for continuous variables and the Fisher exact test for categorical variables. Change in MDI and NMS from 12 to 30 mo was determined using paired t tests. To determine whether the severity of cerebral injury and the severity of neonatal systemic derangement in the outcome groups differed, 1H-MRS ratios and SNAP-PE scores of the groups were compared using the nonparametric test for trends across ordered groups.
Logistic regression was used to compare the following predictor variables in the normal and deteriorated groups: (1) 1H-MRS metabolite ratios, (2) SNAP-PE scores, and (3) total household income. This was done to determine whether deterioration from 12 to 30 mo of age could be attributed to more severe neonatal cerebral injury, more severe neonatal systemic derangement, or to socioeconomic factors.
RESULTS
Fifteen infants (40%) were classified as normal, 11 (30%) as abnormal, and 11 (30%) as deteriorated (Table 1). The deterioration observed in 11 infants was primarily cognitive (Tables 1 and 2). At 12 mo of age, the normal and deteriorated groups did not differ in mean MDI (p = 0.5) or in the distribution of NMS (p = 0.7). At 30 mo of age, the normal and deteriorated groups differed in mean MDI (p < 0.00001), but not in the distribution of NMS (p = 0.9). All of the deteriorated infants had a decrease in MDI from 12 to 30 mo and one of these children also had deterioration in NMS from 2 to 3. The mean MDI decreased only in the deteriorated group (p < 0.00001), and did not change in the other two groups (both p > 0.5).
MRI scores were distributed differently in the three groups on the T2-weighted sequences in the BN (p = 0.01) and IBZ (p = 0.03), with more severe abnormalities in the abnormal group (Table 3). The T1 scores did not differ among the three groups in the BN (p = 0.07) or IBZ (p = 0.3). The MRI scores were not different in the normal and deteriorated groups in the BN or IBZ (all p > 0.2) (Table 3).
NAA/choline differed among the three groups in BN (p ≤ 0.001) and IBZ (p = 0.04), with lowest values in the abnormal group and highest values in the normal group (Table 4, and Figs. 1 and 2). Lactate/choline differed among the three groups in BN (p = 0.01) and IBZ (p = 0.05), with highest values in the abnormal and lowest values in the normal group (Figs. 1 and 2). Similarly, SNAP-PE differed across the three outcome groups, with highest scores in the abnormal group and lowest scores in the normal group (p = 0.01) (Fig. 3).
Box plots of cerebral metabolites by outcome group. (A) BN NAA/choline differed among the three groups with lowest values in the abnormal group and highest values in the normal group (p ≤ 0.001). (B) BN lactate/choline differed among the three groups with highest values in the abnormal and lowest values in the normal group (p = 0.01). (C) IBZ NAA/choline differed among the three groups with lowest values in the abnormal group and highest values in the normal group (p = 0.04). (D) IBZ lactate/choline differed among the three groups with highest values in the abnormal and lowest values in the normal group (p = 0.05).
In comparisons aimed at distinguishing the normal group from the deteriorated group based on neonatal predictor variables, there was no significant difference, but a trend for lower BN NAA/choline in the deteriorated group compared with the normal group [odds ratio 663.7 (95% confidence interval [CI]: 0.38–1,161,336), p = 0.08]. As BN NAA/choline was most strongly associated with outcome status when comparing the three groups, only this 1H-MRS variable was tested to distinguish the normal group from the deteriorated group.
SNAP-PE, the other neonatal predictor variable, was not significantly different between the normal group and the deteriorated group [odds ratio 0.91 (95% CI: 0.79–1.04), p = 0.18].
To determine whether deterioration is associated with socioeconomic factors, we compared the household income of the normal and deteriorated groups using logistic regression (Table 5). The odds of deterioration, if normal at 12 mo, increased by a factor of 5.1 (95% CI: 1.3–19.8;p = 0.02), with each decrease in one of four household income strata (n = 21). To determine whether the effect of household income on the odds of deterioration is independent of BN NAA/choline, a multivariate logistic regression model was used. The results of this model did not differ meaningfully from the univariate analysis. The odds of deterioration, if normal at 12 mo, increased by a factor of 5.1 (95% CI: 1.3–20.3;p = 0.02) with each decrease in one of four household income strata, whereas lower basal nuclei NAA/choline was not significantly associated with deterioration [odds ratio 17.3 (95% CI: 0.0009–319548), p = 0.57]. Given the strong association of household income with normal and deteriorated outcomes, the lack of meaningful univariate association with SNAP-PE in these groups, and our sample size, SNAP-PE was not included in the multivariate regression model.
Only one deteriorated infant (patient 11 in Table 2) had a decline in MDI that was possibly within the expected error of the test. This infant was in the income group $35,000–$75,000, and removing him from the analysis did not significantly alter the results. Of the 15 normal infants, two had improved from abnormal at 12 mo of age. One of these infants, with an MDI of 82 and NMS of 0 at 12 mo, had an annual household income ≥$75,000, and the other, with an MDI of 74 and NMS of 0 at 12 mo, had an annual household income between $35,000 and $75,000.
At 30 mo of age, eight infants in the abnormal group, one infant in the normal group, and none in the deteriorated group were receiving rehabilitation therapy.
DISCUSSION
After perinatal depression in term newborns, the relative cerebral concentrations of NAA and lactate are significantly associated with outcome at 30 mo of age. This is consistent with the observation by this group and others that abnormalities of lactate and, more variably, NAA are associated with neurodevelopmental outcome at 12 mo of age (1, 2, 11–16), although this association was not found by others. Decreases and increases in NAA reflect neuronal integrity (17–20). As diminished NAA/choline after perinatal depression was most strongly associated with outcome at 30 mo of age, our findings suggest that abnormality of neuronal integrity is the most important neonatal predictor of 30-mo outcome.
We have previously reported in this cohort that the most consistent abnormality associated with neurodevelopmental impairment at 12 mo of age was the presence of a lactate peak (2). Persistent increase in lactate after birth asphyxia (>1 mo) may be seen in infants with abnormal neurodevelopmental outcome at 12 mo of age (21). Abnormalities of lactate reflect an ongoing metabolic disturbance (e.g. secondary to hypoxic-ischemic injury), or the presence of inflammatory cells (19, 22). Lactate may reflect more acute metabolic stress and, although more strongly associated with 12-mo outcome than NAA, may be less important for the prediction of longer-term neurodevelopmental outcome. Another factor may be the age of the patient at the time of the 1H-MRS (2). Our experience in human newborns (unreported data) and that of others in neonatal piglets (23) suggests that, after peaking during the second or third day, the size of the lactate peak diminishes during the first 2 wk after birth, and may become normal; thus, patients studied after the age of 3 d may have deceptively low lactate levels. In contrast, NAA concentration diminishes during the first few days after injury and thereafter remains lower than normal. We speculate that the fact that our patients were studied at a median age of 6 d may explain the increased sensitivity of the NAA measurements.
The deterioration in cognitive outcome is closely associated with lower socioeconomic condition, even when controlling for the severity of neuronal injury as measured by NAA/choline. Criteria available in the neonatal period (lower NAA/choline, more severe structural brain injury on MRI, or more severe systemic derangement) or at 12 mo of age (lower NMS) could not adequately predict later neurodevelopmental deterioration in infancy. Developmental disabilities in ICN graduates, including term infants with hypoxic-ischemic encephalopathy, have been associated with sociodemographic variables (poverty, race, maternal education, and gender) by several other investigators (4, 5, 24). These variables seem to have greatest effects on mild educational disabilities (25). In our abnormal group, the group with the most severe developmental disabilities, income strata were evenly distributed. This is consistent with the previous observation that severe developmental impairments are more closely associated with perinatal factors (25).
The group of most clinical concern in our cohort was those infants who were classified as normal at 12 mo of age who were later classified as abnormal. It is unlikely that this observation is an artifact of the test procedures. The MDI of the BSID is a stable measure of infant development in high-risk ICN survivors (26). Others have demonstrated that repeated assessment with the BSID did not bias the MDI, and that scores at 4 mo and 28 mo with repeated assessment were equivalent (27). This “repeated assessment” bias would not explain our findings, as the direction of this bias would be expected to normalize the scores of impaired infants on follow-up, thereby reducing any apparent “deterioration.” An alternative explanation for the observed deterioration is that abnormal cognition became apparent only at 30 mo of age in the deteriorated group. Although the MDI at 30 mo is more enriched in verbal items than at 12 mo, we would not expect “deterioration” in only a subset of our cohort. Also, as the MR scores did not differ in the normal and deteriorated groups in the BN or IBZ, it is unlikely that the deterioration was due to late manifestations of frontal white-matter injury where impaired cognition with relatively preserved motor function would be expected.
The relationship between socioeconomic status and neurodevelopmental outcome is multifaceted and may relate, at least in part, to environmental stimulation and access to rehabilitation therapy. No infants in the deteriorated group were receiving rehabilitation therapy at 30 mo of age. The importance of recognizing the group of children at high risk of deterioration after 12 mo of age is underscored by the potential gains of early developmental intervention in those children from a lower socioeconomic environment (28). These interventions, as demonstrated in low-birth-weight preterm children, may be most effective in improving developmental outcomes in those born to mothers with high school education or less (28). In term small-for-gestational-age infants and preterm infants, higher maternal language skills, a positive home environment, and attendance at preschool were associated with a significant increase in the mean intelligence quotient (29). The negative effects of low socioeconomic status may overshadow the impact of pre- or perinatal hypoxic-ischemic injury in those children with mild to moderate impairments.
After perinatal depression in term newborns, normal neurodevelopment at 12 mo of age does not ensure a normal long-term outcome. Overall, given the prevalence and implications of low socioeconomic status, it is incumbent on the clinician to identify these high-risk infants so that they continue to be followed after 12 mo of age and are targeted for appropriate developmental services.
Abbreviations
- BN:
-
basal nuclei
- IBZ:
-
intervascular boundary zones
- ICN:
-
intensive care nursery
- MDI:
-
mental development index
- MR:
-
magnetic resonance
- NAA:
-
N-acetylaspartate
- NMS:
-
neuromotor score
- 1H-MRS:
-
proton magnetic resonance spectroscopy
References
Amess PN, Penrice J, Wylezinska M, Lorek A, Townsend J, Wyatt JS, Amiel-Tison C, Cady EB, Stewart A 1999 Early brain proton magnetic resonance spectroscopy neonatal neurology related to neurodevelopmental outcome at 1 year in term infants after presumed hypoxic-ischaemic brain injury. Dev Med Child Neurol 41: 436–445
Barkovich AJ, Baranski K, Vigneron D, Partridge JC, Hallam DK, Hajnal BL, Ferriero DM 1999 Proton MR spectroscopy for the evaluation of brain injury in asphyxiated, term neonates. AJNR Am J Neuroradiol 20: 1399–1405
Holshouser BA, Ashwal S, Luh GY, Shu S, Kahlon S, Auld KL, Tomasi LG, Perkin RM, Hinshaw DB, Jr 1997 Proton MR spectroscopy after acute central nervous system injury: outcome prediction in neonates, infants, children. Radiology 202: 487–496
Piecuch RE, Leonard CH, Cooper BA, Sehring SA 1997 Outcome of extremely low birth weight infants (500 to 999 grams) over a 12-year period. Pediatrics 100: 633–639
Resnick MB, Gomatam SV, Carter RL, Ariet M, Roth J, Kilgore KL, Bucciarelli RL, Mahan CS, Curran JS, Eitzman DV 1998 Educational disabilities of neonatal intensive care graduates. Pediatrics 102: 308–314
Barkovich AJ, Hajnal BL, Vigneron D, Sola A, Partridge JC, Allen F, Ferriero DM 1998 Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol 19: 143–149
Richardson DK, Phibbs CS, Gray JE, McCormick MC, Workman-Daniels K, Goldmann DA 1993 Birth weight illness severity: independent predictors of neonatal mortality. Pediatrics 91: 969–975
Richardson DK, Gray JE, McCormick MC, Workman K, Goldmann DA 1993 Score for Neonatal Acute Physiology: a physiologic severity index for neonatal intensive care. Pediatrics 91: 617–623
Bayley N 1993 The Bayley Scales of Infant Development II. New York Psychological Corporation, New York
Hajnal BL, Sahebkar-Moghaddam F, Barnwell AJ, Barkovich AJ, Ferriero DM 1999 Early prediction of neurologic outcome after perinatal depression. Pediatr Neurol 21: 788–793
Ashwal S, Holshouser BA, Tomasi LG, Shu S, Perkin RM, Nystrom GA, Hinshaw DB, Jr 1997 1H-magnetic resonance spectroscopy-determined cerebral lactate poor neurological outcomes in children with central nervous system disease. Ann Neurol 41: 470–481
Hanrahan JD, Sargentoni J, Azzopardi D, Manji K, Cowan FM, Rutherford MA, Cox IJ, Bell JD, Bryant DJ, Edwards AD 1996 Cerebral metabolism within 18 hours of birth asphyxia: a proton magnetic resonance spectroscopy study. Pediatr Res 39: 584–590
Holshouser BA, Ashwal S, Shu S, Hinshaw DB Jr 2000 Proton MR spectroscopy in children with acute brain injury: comparison of short long echo time acquisitions. J Magn Reson Imaging 11: 9–19
Shu SK, Ashwal S, Holshouser BA, Nystrom G, Hinshaw DB Jr 1997 Prognostic value of 1H-MRS in perinatal CNS insults. Pediatr Neurol 17: 309–318
Groenendaal F, Veenhoven RH, van der Grond J, Jansen GH, Witkamp TD, de Vries LS 1994 Cerebral lactate N-acetyl-aspartate/choline ratios in asphyxiated full-term neonates demonstrated in vivo using proton magnetic resonance spectroscopy. Pediatr Res 35: 148–151
Penrice J, Cady EB, Lorek A, Wylezinska M, Amess PN, Aldridge RF, Stewart A, Wyatt JS, Reynolds EO 1996 Proton magnetic resonance spectroscopy of the brain in normal preterm term infants, early changes after perinatal hypoxia-ischemia. Pediatr Res 40: 6–14
Hüppi PS, Fusch C, Boesch C, Burri R, Bossi E, Amato M, Herschkowitz N 1995 Regional metabolic assessment of human brain during development by proton magnetic resonance spectroscopy in vivo and by high-performance liquid chromatography/gas chromatography in autopsy tissue. Pediatr Res 37: 145–150
Kreis R, Ernst T, Ross BD 1993 Development of the human brain: in vivo quantification of metabolite water content with proton magnetic resonance spectroscopy. Magn Reson Med 30: 424–437
Novotny E, Ashwal S, Shevell M 1998 Proton magnetic resonance spectroscopy: an emerging technology in pediatric neurology research. Pediatr Res 44: 1–10
Toft PB, Christiansen P, Pryds O, Lou HC, Henriksen O 1994 T1, T2, concentrations of brain metabolites in neonates adolescents estimated with H-1 MR spectroscopy. J Magn Reson Imaging 4: 1–5
Hanrahan JD, Cox IJ, Edwards AD, Cowan FM, Sargentoni J, Bell JD, Bryant DJ, Rutherford MA, Azzopardi D 1998 Persistent increases in cerebral lactate concentration after birth asphyxia. Pediatr Res 44: 304–311
Petroff OA, Graham GD, Blamire AM, al-Rayess M, Rothman DL, Fayad PB, Brass LM, Shulman RG, Prichard JW 1992 Spectroscopic imaging of stroke in humans: histopathology correlates of spectral changes. Neurology 42: 1349–1354
Penrice J, Lorek A, Cady EB, Amess PN, Wylezinska M, Cooper CE, D'Souza P, Brown GC, Kirkbride V, Edwards AD, Wyatt JS, Reynolds EO 1997 Proton magnetic resonance spectroscopy of the brain during acute hypoxia-ischemia delayed cerebral energy failure in the newborn piglet. Pediatr Res 41: 795–802
Robertson C, Finer N 1985 Term infants with hypoxic-ischemic encephalopathy: outcome at 3.5 years. Dev Med Child Neurol 27: 473–484
Resnick MB, Gueorguieva RV, Carter RL, Ariet M, Sun Y, Roth J, Bucciarelli RL, Curran JS, Mahan CS 1999 The impact of low birth weight, perinatal conditions, sociodemographic factors on educational outcome in kindergarten. Pediatrics 104: e74
Crowe TK, Deitz JC, Bennett FC 1987 The relationship between the Bayley Scales of Infant Development preschool gross motor cognitive performance. Am J Occup Ther 41: 374–378
Haskins R, Ramey CT, Stedman DJ, Blacher-Dixon J, Pierce JE 1978 Effects of repeated assessment on standardized test performance by infants. Am J Ment Defic 83: 233–239
Brooks-Gunn J, Gross RT, Kraemer HC, Spiker D, Shapiro S 1992 Enhancing the cognitive outcomes of low birth weight, premature infants: for whom is the intervention most effective?. Pediatrics 89: 1209–1215
Goldenberg RL, DuBard MB, Cliver SP, Nelson KG, Blankson K, Ramey SL, Herman A 1996 Pregnancy outcome intelligence at age five years. Am J Obstet Gynecol 175: 1511–1515
Acknowledgements
The authors thank Amy Ferrer Rogers, project manager, Faith Allen, data manager, and the neonatal nurses of the Pediatric Clinical Research Center for their expertise.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Institutes of Health (NIH) Grant NS35902 and NIH Pediatric Clinical Research Center Grant M01-RR01271 (D.M.F., N.N., A.J.B.). S.P.M. is supported by the Canadian Institutes of Health Research Clinician Scientist Program (Phase 1) and the Glaser Pediatric Research Network Fellowship Award.
Rights and permissions
About this article
Cite this article
Miller, S., Newton, N., Ferriero, D. et al. Predictors of 30-Month Outcome after Perinatal Depression: Role of Proton MRS and Socioeconomic Factors. Pediatr Res 52, 71–77 (2002). https://doi.org/10.1203/00006450-200207000-00014
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200207000-00014
This article is cited by
-
The predictive value of MRI scores for neurodevelopmental outcome in infants with neonatal encephalopathy
Pediatric Research (2024)
-
Preoperative serum cortisone levels are associated with cognition in preschool-aged children with tetralogy of Fallot after corrective surgery: new evidence from human populations and mice
World Journal of Pediatrics (2024)
-
How well does neonatal neuroimaging correlate with neurodevelopmental outcomes in infants with hypoxic-ischemic encephalopathy?
Pediatric Research (2023)
-
Brain proton magnetic resonance spectroscopy and neurodevelopment after preterm birth: a systematic review
Pediatric Research (2022)
-
Magnetic resonance spectroscopy brain metabolites at term and 3-year neurodevelopmental outcomes in very preterm infants
Pediatric Research (2022)