Abstract
The application of targeted gene inactivation methodologies to the study of late fetal development and control of the timing for parturition in mice has yielded insight into the mechanisms that enhance fetal survival. An essential role for glucocorticoids in promoting lung maturation sufficient for viability ex utero before the onset of normal parturition has been demonstrated in corticotropin-releasing hormone–deficient mice. In contrast, maternal deficiency in the prostaglandin synthetic enzyme cyclooxygenase-1 results in the markedly delayed onset of labor and fetal demise because of postdates gestation. The complex interplay of factors that govern the onset of labor is highlighted by mice deficient in both cyclooxygenase-1 and oxytocin. Whereas mice deficient in oxytocin demonstrate normal parturition, simultaneous cyclooxygenase-1 and oxytocin deficiency rescues the delayed onset of labor found in cyclooxygenase-1 knockout mice but results in the prolonged duration of labor. The consequences of complete deficiency of molecules involved in parturition in mice suggest novel interventions for human preterm labor.
Similar content being viewed by others
Main
Understanding the mechanisms coordinating the rate of fetal maturation with the length of gestation and the initiation of labor are of fundamental biologic interest and substantial medical importance. Preterm labor continues to be a frequent complication of pregnancy, with sequelae for premature infants not yet prepared for viability ex utero including hyaline membrane disease, intraventricular hemorrhage, necrotizing enterocolitis, growth failure, and sepsis (1, 2). The support of these premature newborns exacts significant emotional and financial costs, not only in terms of the acute complications and intensive care management they require, but also because of the development of chronic sequelae such as bronchopulmonary dysplasia and cerebral palsy. For these reasons, intervention intended to prevent or significantly delay premature labor would be of great utility.
Characterization of the initiators of human labor has proven difficult. In part, this difficulty stems from the limited ability to extrapolate mechanisms from nonprimate systems to humans. For example, in the sheep, activation of the fetal hypothalamic-pituitary-adrenal axis, and the accompanying GC surge, clearly precipitates labor (3). In this species, rising serum cortisol concentration induces 17-hydroxylase/17, 20 lyase activity, which reduces progesterone production, resulting in increased PG synthesis and labor (4). In women, this mechanism is not maintained (5). GC administration does not precipitate labor, although recent studies suggest that placental CRH, which could augment GC production, may contribute to the timing of parturition (6–8). Administration of exogenous GC to fetal sheep also results in acceleration of lung maturation, suggesting that the endogenous GC rise serves to promote both fetal maturation and the onset of labor (9, 10).
The application of molecular genetic techniques that allow mutational analysis in vivo shows promise for elucidating relevant molecular pathways that control parturition. To date, transgenic and KO mice are the most readily accessible mammalian systems for such studies (11–13). The maintenance of pregnancy in the mouse differs from that in humans in that progesterone and estradiol are synthesized in the corpus luteum of the ovary throughout gestation, whereas luteal function in the human is necessary to maintain pregnancy for only the first trimester (14, 15). Additionally, the termination of pregnancy in the mouse is precipitated by a decline in serum progesterone, whereas the termination of pregnancy in humans is not. Instead, local modulation of progesterone synthesis or action within the uterus and fetal membranes has been suggested as a mechanism by which diminished progesterone action could still result in labor in humans (16–18). Nevertheless, conserved regulation and critical functions for several of the same molecular pathways in mouse and human labor have emerged, as would be expected for a process as fundamental as parturition. Targeted inactivation studies using homologous recombination in ES cells (13) to generate mice completely deficient in specific neuropeptides and interacting molecules have recently implicated the importance of these systems in the maintenance of gestation and the control of parturition in humans. The results of three such systems will be reviewed.
CORTICOTROPIN-RELEASING HORMONE
Our initial investigations of neuropeptides in the modulation of the timing of parturition and regulation of fetal maturation centered on defining the contribution of CRH to these processes. CRH is a 41-amino acid peptide initially isolated by Vale et al. (19, 20) on the basis of its ability to augment ACTH release from the pituitary and modulate the function of the hypothalamic-pituitary-adrenal axis. In addition to CRH localized to paraventricular nucleus of the hypothalamus and destined for release into the hypophyseal portal blood supply, this peptide has been found in other brain regions, as well as nonneural sites (21–23). Importantly, CRH in human serum has been found to increase dramatically during pregnancy because of high-level expression by the placenta (6, 8, 24, 25). Moreover, CRH-binding protein, which limits the bioactivity of circulating CRH, decreases in abundance late in gestation (26, 27). The timing of the placental increase in CRH synthesis, together with increasing CRH bioactivity, has been implicated as an important contributor to the timing of labor in humans, although the function of CRH during parturition remains uncertain (6, 8).
To directly examine the role of CRH during murine pregnancy, and GC whose synthesis would be altered as a consequence of CRH deficiency, we generated mice homozygous for CRH deficiency by homologous recombination in ES cells (23). Mice homozygous for CRH deficiency derived from heterozygote matings were present in the expected Mendelian ratios and appeared healthy (28). Despite this normal appearance, CRH KO mice exhibited adrenal atrophy and deficient GC production (Fig. 1). Female CRH KO mice generated serum GC (corticosterone in mice) concentrations approximately 30% of WT concentrations after stress, and the male CRH KO mice exhibited even more profound adrenal insufficiency, displaying no significant increase in their serum corticosterone above basal concentrations after a variety of stresses including restraint, fasting, and ether (28).
Adrenal hypoplasia in CRH KO mice. Hematoxylin-eosin stained sections of adrenals from 8-wk-old CRH KO (a) or WT (b) mice are shown. The normal strandlike appearance of the adrenal cortex, predominantly the GC-producing zona fasciculata (ZF), is seen in the WT adrenal, whereas the CRH KO has very little ZF between the adrenal medulla (M) and zona glomerulosa (ZG), located beneath the adrenal capsule. Reproduced with permission from Muglia et al. (28).
CRH KO male and female mice proved fertile. Despite the evidence of placental CRH serving an important function in human pregnancy (6) and fetal hypothalamic CRH serving an important function in ovine pregnancy (29), the completely CRH-deficient gestations resulted in a normal number of pups delivered at the expected time. The normal timing for labor in the CRH KO pregnancies does not exclude a role for CRH in human pregnancy, because mouse placenta does not synthesize appreciable amounts of CRH (23). In striking contrast to CRH KO progeny of heterozygote matings, however, all pups arising from KO × KO matings died on the first day of life (28). Inasmuch as CRH KO progeny of heterozygote matings are viable, the heterozygous mother must provide a factor that crosses the placenta and rescues the homozygous KO fetus. Maternofetal transfer across the placenta of CRH, a peptide hormone, in amounts sufficient to rescue the KO progeny of heterozygote matings, would be unlikely. Therefore, we tested the hypothesis that GC, present in normal concentrations in the heterozygous mother and deficient in the CRH KO dams, crossed the placenta and restored viability to pups of KO × KO matings. Indeed, administration of corticosterone in the drinking water to gravid CRH KO females resulted in birth of viable progeny (28).
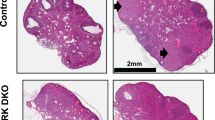
To determine the basis of CRH and resultant glucocorticoid deficiency leading to fetal demise, we performed histologic analysis on the progeny of timed KO × KO and WT matings. These studies revealed a failure of lung morphologic maturation after day 16.5 of mouse gestation, corresponding to the progression from the early to the late canalicular phase of pulmonary development (Fig. 2) (28, 30). The CRH KO lung was hypercellular, as reflected by increased wet weight, dry weight, and DNA content in comparison to WT lung at 18.5 d gestation. This hypercellularity in the CRH KO lung did not result from impaired apoptosis, but rather increased cell proliferation as determined by sustained proliferating cell nuclear antigen immunoreactivity late in gestation (30).
Lung morphogenesis in CRH KO and WT mice. Hematoxylin-eosin stained sections of lung from fetuses of 14.5 through 18.5 d gestation photographed at ×200 magnification are shown. (a) WT 14.5 d; (b) CRH KO 14.5 d; (c) WT 16.5 d; (d ) CRH KO 16.5 d; (e) WT 17.5 d; (f) CRH KO 17.5 d; (g) WT 18.5 d; (h) CRH KO 18.5 d. A divergence in architecture is apparent at day 17.5, with the CRH KO maintaining a dense, pseudoglandular appearance. Reproduced with permission from Muglia et al. (30).
Despite earlier studies on pulmonary development that implicated endogenous GC as a modulator of surfactant apoprotein gene expression, our findings in the CRH KO mice demonstrated no substantial differences in either surfactant apoprotein or lipid biosynthesis (30). These findings, which are consistent with the normal levels of the surfactant apoprotein mRNAs in the GC receptor KO mice at birth (31), argue against an absolute requirement of GC for surfactant apoprotein gene expression and suggest that the essential effects of GC on fetal lung maturation are on cell proliferation and architectural maturation. This concept is further supported by the analysis of markers of epithelial differentiation in the CRH KO mice, which again reveal that the fatality observed in CRH-deficient mice results directly from an overall delay in the timing of pulmonary maturation secondary to GC insufficiency (30). Taken together, the striking discordance between the rate of fetal maturation and the onset of labor in the CRH-deficient mice underscores the essential role of GC in the timing of fetal development such that viability ex utero is achieved.
OXYTOCIN
OT, a cyclic nonapeptide produced primarily in the hypothalamic paraventricular and supraoptic nuclei, augments uterine contractions and endometrial and amnion PG production when given to humans in either pharmacologic or physiologic doses (32). Additionally, the abundance of uterine myometrial OT receptors increases more than 10-fold as pregnancy nears term (33). Thus, OT has been considered an important facilitator of labor at term. Other studies, however, have shown no increase in plasma OT concentrations before the onset of labor (34, 35). Moreover, OT antagonists fail to alter the onset of labor (36, 37), and humans with damage to the posterior pituitary, the site of release of OT synthesized in the hypothalamus, have been observed to progress through labor normally. Confusing the determination for or against a necessary role for OT in parturition is its paracrine production and action within the uterus and decidua, a setting in which blockade by an antagonist could be incomplete (33).
Using a strategy similar to that in creation of CRH KO mice, we (38) and other groups (39, 40) generated mice with OT deficiency by homologous recombination in ES cells. Most surprisingly, gravid OT KO females, including those that had been mated with OT KO males, initiated parturition at the expected time of 19.5 d of gestation and did not demonstrate prolongation of active labor. Therefore, neither maternal nor fetal OT is required for normal labor to occur in the mouse. Progeny arising from OT KO females die in the first days of life because the OT KO females do not lactate normally (39, 40). As expected from the myoepithelial localization of OT receptors, this lactation defect results from defective milk letdown rather than production.
CYCLOOXYGENASE-1
One pathway that, like OT, can pharmacologically accelerate labor is that of PG synthesis. PG induce luteolysis, augment uterine contractions, and promote the controlled inflammatory response that results in dilatation and thinning of the cervix and disruption of the connective tissue of the decidua (41). Indeed, the administration of nonsteroidal anti-inflammatory drugs, which block PG production, have proven efficacious in attenuating the progression of term and preterm labor in animal model systems (42, 43), as well as in human studies (44, 45). Not only do amniotic fluid concentrations of PG and other cytokines rise as labor proceeds (46, 47), but it has recently been demonstrated that the increase in amniotic fluid PG occurs before the onset of labor in humans (48).
All PG synthesis starts with the conversion of arachidonic acid to PGH2. This production is catalyzed by COX (PGH synthase), an enzyme with two isoforms, designated COX-1 and COX-2. These COX isoforms share similar structures and are the primary sites of action of the nonsteroidal anti-inflammatory drugs (49, 50), but differ significantly in their pattern of regulation. COX-1 is constitutively expressed in most tissue types. Although stimulation of expression can occur in certain cell types after growth factor exposure, little regulation has in general been demonstrated, including in extrafetal membranes during parturition (51–53). COX-2, in contrast, is in general undetectable in most tissues but can be expressed at high concentrations in macrophages and other inflammatory cell types after cytokine exposure (51, 54). COX-2 is expressed in the amnion, and rises in cultured amnion cells in response to IL-1β administration (55).
Molecular genetic studies on PG synthetic enzymes and PG receptors have confirmed the central role of PG in murine parturition. PGF2α receptor- (56) and cytoplasmic phospholipase A2- (57) deficient mice fail to initiate labor because of impaired luteolysis and persistent progesterone production. Because of this failure of luteolysis, myometrial OT receptors are not induced (56). In addition to inhibiting induction of OT receptor expression in the uterus, the sustained production of progesterone by the corpus luteum would be anticipated to directly impair OT receptor activity (58). If OT receptors are induced in these mice by ovariectomy, however, labor is initiated and progresses normally (56). Thus, PGF2α appears to be required for luteolysis in mice, but is not essential for uterine contractions if a fall in progesterone occurs by other means. Although PGE2 action has been implicated in closure of the ductus arteriosus in mice deficient in one of the PGE2 receptors, EP4 (59), and fertilization of the ovum in EP2 receptor-deficient mice (60, 61), parturition defects have not been described in any mouse mutants deficient in the four known PGE2 receptors (62).
To determine which of the COX isoforms generates the PGF2α necessary for the onset and progression of labor at term in mice, we evaluated the parturition phenotype in COX-1 KO mice. The initial characterization of COX-1 KO mice indicated that both males and females were healthy and resisted arachidonic acid-induced inflammation (63). Pregnancies arising from COX-1 KO male × COX-1 KO female matings, however, culminated in delivery of nonviable fetuses (63). To determine whether fetal demise at term in the COX-1 KO mice was caused by an abnormality in the onset or progression of labor, we established timed pregnancies of COX-1 KO females mated with COX-1 KO or COX-1-intact males (38). In marked contrast to the onset of labor at day 19.5 of gestation in WT mice, COX-1 KO females delivered their litters at day 21.5–22.0. Administration of PGF2α to the gravid COX-1 KO females at day 18.5–19.5 resulted in delivery of viable pups, demonstrating that fetal demise was caused by postdates gestation and not by a developmental abnormality arising from COX-1 deficiency. Blastocyst transfer of COX-1 KO or WT embryos to pseudopregnant WT females further demonstrated that fetal COX-1 was not required for the normal onset of labor (38). Thus, these studies revealed that maternal COX-1 is necessary and sufficient for normal murine labor.
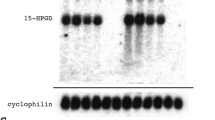
Biochemical analyses of the mechanism of action of COX-1 showed that COX-1 was induced in the uterine decidua at high concentrations in late mouse gestation and accounted for the vast majority of PGF2α production (38) (Fig. 3). The induction of COX-1 mRNA was surprising given the usual housekeeping activity this isoform is thought to mediate. The insufficient PGF2α production in the COX-1 KO mice inefficiently initiated luteolysis and induction of OT receptors, consistent with studies in the PGF2α receptor and cytoplasmic phospholipase A2 KO mice (56, 57). The poor ovulatory frequency and impaired blastocyst implantation found in COX-2 KO mice (64) has thus far precluded genetic determination of the consequences of complete COX-2 deficiency for the onset and progression of term labor in mice.
COX-1 mRNA induction and uterine PGF2α during pregnancy in mice. (a) Northern blot analysis of total uterine RNA. Samples from nongravid (NG) or pregnant females of the gestational age in days shown above the corresponding lanes were hybridized to COX-1 or cyclophilin (CYC) probes. (b) Impaired uterine PGF2α production in COX-1 KO and COX-1 KO/OT KO mice. PG were extracted from uteri of the indicated genotypes, and PGF2α determined by ELISA. Assay specificity was confirmed by administration of indomethacin (Indo) to a subset of WT mice at day 15.5 of gestation to suppress PG production. Data are presented as mean, with day 19 samples ± SEM. *p < 0.05 vs WT at day 19. Reproduced with permission from Gross et al. (38).
Genetic analyses in mice facilitate the ability to dissect redundant in vivo pathways by generation of animals with combined deficiencies. To determine whether redundant OT and PG action on the myometrium allowed both the normal time of onset and normal duration of labor in OT KO mice, and the normal duration of labor despite the delayed onset of labor in COX-1 KO mice, we generated mice deficient in both COX-1 and OT (COX-1 KO/OT KO) (38). We hypothesized that with deficiency in both PG and OT activity, not only would there be a delay in the initiation of labor, but also a prolongation in the duration of labor. In part, this expectation was fulfilled with the COX-1 KO/OT KO females demonstrating a markedly prolonged duration of labor with delivery of pups occurring during a 2-d period. Surprisingly, OT deficiency restored the onset of labor to normal (day 19.8). COX-1 KO/OT KO mice underwent luteolysis in a manner similar to WT mice, although they did not require the usual increase in PG found in WT mice before the onset of parturition (38) (Fig. 4). These findings suggest that OT not only facilitates uterine contractions, but also serves to maintain corpus luteum function in late gestation. Whether OT action occurs directly on the corpus luteum, or results from modulation of other peptides, such as prolactin, known to affect luteal function, is currently under investigation.
Corpus luteum histology at day 19 of gestation. Corpora lutea of WT and COX-1 KO/OT KO mice demonstrate an amorphous cellular appearance and disruption of luteal architecture consistent with luteolysis. In contrast, the COX-1 KO corpus luteum, which has not undergone luteolysis, maintains prominent vascular spaces, a uniform cellular appearance, and an organized luteal architecture (×200 magnification). Reproduced with permission from Gross et al. (38).
SUMMARY AND FUTURE DIRECTIONS
The molecular genetic analysis of the control of parturition in mice with targeted gene mutations has allowed dissection of the mechanism of labor initiation in this species (Fig. 5). Arachidonic acid release from cellular lipid stores by the action of cytoplasmic phospholipase A2 provides substrate to COX-1 for generation of PGF2α. Induction of COX-1 in the uterus during gestation then results in a dramatic increase in PGF2α late in gestation. The increased PGF2α, via action on PGF2α receptors on the corpus luteum, causes luteolysis and a decrease in serum progesterone. The fall in progesterone induces a state of increased uterine contractility, by diminished direct antagonism of OT receptor signaling, and induction of OT receptors, contractile PG receptors, gap junctions, and other myometrial proteins that increase sensitivity to uterotonic agents. OT and PG actions at the level of the myometrium appear to be at least in part redundant inasmuch as only in mice with combined OT and COX-1 deficiency is the duration of labor prolonged (38). The induction of COX-1 at least 3 d before the onset of labor (38) indicates that the process of parturition is set in motion well before the appearance of the known indicators of luteolysis and labor. Further analysis of COX-1 regulation should prove valuable in determining which maternal or fetal signals are involved in the activation of labor at even earlier times during gestation and thus provide new insight into the clock mechanism of parturition.
Scheme for parturition in mice. Arachidonic acid released from membrane phospholipids by the action of cytoplasmic phospholipase A2 (cPLA2) serves as substrate for COX-1 to generate PGH2. PGH2 is converted by the action of PGF synthase to PGF2α. Increased uterine (and perhaps ovarian) generation of PGF2α late in gestation causes involution of the ovarian corpus luteum by action on the PGF2α receptor. The resulting fall in progesterone increases uterine contractility and promotes labor by decreased direct antagonism of the OT receptor, and induction of OT receptors, contractile PG receptors, and gap junctions, among other myometrial proteins.
What do these experiments teach us about the control of human parturition? Extrapolation of the mechanism by which PG promote parturition in mice to humans is not straightforward (14, 15), although PG are potent modulators of labor in both species. The corpus luteum in humans does not actively contribute to progesterone production in late gestation, and progesterone does not fall before the onset of labor. Is there a functional equivalent in the human to the rodent corpus luteum, e.g. the placenta or fetal adrenal? Our studies suggest that the analysis of the consequences of PGF2α action on these structures will be useful in this regard. Several studies have investigated which of the COX isoforms is essential for the rise in PG in late human gestation, with the only clear increase occurring in COX-2 from extraembryonic tissues (53, 65–67). More important, determination of the isoform critical for imparting preterm labor in humans remains controversial (67, 68). Thus far, no naturally occurring or targeted mutations imparting preterm labor in the mouse have been described. As novel candidate genes implicated in the pathogenesis of human preterm labor are identified, in vivo genetic analysis in the mouse will greatly accelerate determining their mechanism of action. Inflammatory models of preterm labor in mice, such as lipopolysaccharide administration (43, 69), have been used and may provide a reasonable analog of some forms of preterm labor in humans. Determination of whether COX-1 or COX-2 plays the essential role in inflammation-mediated preterm labor in mice, and ultimately in humans, may allow efficacious therapy by selectively modulating the activity of only one COX isoform. By using appropriate isoform-specific inhibition, preterm labor could be stopped without imparting the many sequelae of nonselective COX blockade if activity of the targeted isoform was not also essential for other aspects of fetal and maternal organ function.
Abbreviations
- CRH:
-
corticotropin-releasing hormone
- COX:
-
cyclooxygenase
- ES:
-
embryonic stem
- GC:
-
glucocorticoid
- KO:
-
knockout
- OT:
-
oxytocin
- PG:
-
prostaglandin
- WT:
-
wild type
References
NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes 1995 Effect of corticosteroids for fetal maturation on perinatal outcomes: NIH Consensus Conference. JAMA 273: 413–418.
Crowley P, Chalmers MJNC 1990 The effects of corticosteroid administration before preterm delivery: an overview of the evidence from controlled trials. Br J Obstet Gynaecol 97: 11–25.
Challis JRG, Brooks AN 1989 Maturation and activation of hypothalamic-pituitary-adrenal function in fetal sheep. Endocr Rev 10: 182–204.
Thorburn GD, Challis JRG 1979 Endocrine control of parturition. Physiol Rev 59: 863–917.
Liggins GC, Forster CS, Grieves SA, Schwartz AL 1977 Control of parturition in man. Biol Reprod 16: 39–56.
McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R 1995 A placental clock controlling the length of human pregnancy. Nat Med 1: 460–463.
Lockwood CJ 1999 Stress-associated preterm delivery: the role of corticotropin-releasing hormone. Am J Obstet Gynecol 180: S264–S266.
Majzoub JA, McGregor JA, Lockwood CJ, Smith R, Taggart MS, Schulkin J 1999 A central theory of preterm and term labor: putative role for corticotropin-releasing hormone. Am J Obstet Gynecol 180: S232–S241.
Liggins GC 1969 Premature delivery of foetal lambs infused with glucocorticoids. J Endocrinol 42: 515–523.
Avery ME 1995 Historical overview of antenatal steroid use. Pediatrics 95: 133–135.
Hogan B, Beddington R, Constantini F, Lacy E 1994 Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Bradley A 1987 Production and analysis of chimeric mice. In: Robertson EJ (ed) Teratocarcinomas and Embryonic Stem Cells: A Practical Approach. IRL Press, Oxford, UK, 113–151.
Majzoub JA, Muglia LJ 1996 Knockout mice. N Engl J Med 334: 904–907.
Challis JRG, Lye SJ 1994 Parturition. In: Knobil E, Neill JD (eds) The Physiology of Reproduction. Raven Press, New York, 985–1031.
Challis JRG 1997 Prostaglandins and reproduction: what do knockouts really tell us?. Nat Med 3: 1326–1327.
Milewich L, Grant NF, Shwarz BE, Chen GT, MacDonald PC 1977 Initiation of human parturition. Obstet Gynecol 50: 45–48.
Mitchell BF, Cruickshank B, McLean D, Challis JRG 1982 Local modulation of progesterone production in human fetal membranes. J Clin Endocrinol Metab 55: 1237–1239.
Casey ML, MacDonald PC 1996 Transforming growth factor-β inhibits progesterone-induced enkephalinase expression in human endometrial stromal cells. J Clin Endocrinol Metab 81: 4022–4027.
Vale W, Spiess J, Rivier C, Rivier J 1981 Characterization of a 41-residue ovine hypothalamic peptide that stimulates the secretion of corticotropin and β-endorphin. Science 213: 1394–1397.
Vale W, Rivier C, Brown MR, Spiess J, Koob G, Swanson L, Bilezikjian L, Bloom F, Rivier J 1983 Chemical and biological characterization of corticotropin releasing factor. Recent Prog Horm Res 39: 245–270.
Grigoriadis D, Jeroux J, De Souza E 1993 Characterization and regulation of corticotropin-releasing factor receptors in the central nervous, endocrine, and immune systems. In: Vale W (ed) Corticotropin Releasing Factor. John Wiley and Sons, Chichester, England, 85–100.
Thompson RC, Seasholtz AF, Herbert E 1987 Rat corticotropin releasing hormone gene: sequence and tissue specific expression. Mol Endocrinol 1: 363–370.
Muglia LJ, Jenkins NA, Gilbert DJ, Copeland NG, Majzoub JA 1994 Expression of the mouse corticotropin-releasing hormone gene in vivo and targeted inactivation in embryonic stem cells. J Clin Invest 93: 2066–2072.
Frim DF, Emanuel RL, Robinson BG, Smas CF, Adler GK, Majzoub JA 1988 Characterization and gestational regulation of preprocorticotropin releasing hormone messenger RNA in human placenta. J Clin Invest 82: 287–292.
Sasaki A, Liotta AS, Luckey MM, Margioris AN, Suda T, Krieger DT 1984 Immunoreactive corticotropin-releasing hormone is present in human maternal plasma during the third trimester of pregnancy. J Clin Endocrinol Metab 59: 812–814.
Linton EA, Perkins AV, Woods RJ, Eben F, Wolfe CD, Behan DP, Potter E, Vale WW, Lowry PJ 1993 Corticotropin releasing hormone-binding protein (CRH-BP): plasma levels decrease during the third trimester of normal human pregnancy. J Clin Endocrinol Metab 76: 260–262.
Petraglia F, Benedetto C, Florio P, D'Ambrogio G, Genazzani AD, Marozio L, Vale W 1995 Effect of corticotropin-releasing factor-binding protein on prostaglandin release from cultured maternal decidua and on contractile activity of human myometrium in vitro. J Clin Endocrinol Metab 80: 3073–3076.
Muglia L, Jacobson L, Dikkes P, Majzoub JA 1995 Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature 373: 427–432.
McDonald TJ, Nathanielsz PW 1991 Bilateral destruction of the fetal paraventricular nuclei prolongs gestation in sheep. Am J Obstet Gynecol 165: 764–770.
Muglia LJ, Bae DS, Brown TT, Vogt SK, Alvarez JG, Sunday ME, Majzoub JA 1999 Proliferation and differentiation defects during lung development in corticotropin-releasing hormone deficient mice. Am J Respir Cell Mol Biol 20: 181–188.
Cole TJ, Blendy JA, Monaghan AP, Kriegelstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schutz G 1995 Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev 9: 1608–1621.
Pickering BT 1989 Oxytocin and its neurophysin. In: DeGroot LJ (ed) Endocrinology. WB Saunders, Philadelphia, 230–239.
Zingg HH, Rozen F, Chu K, Larcher A, Arslan A, Richard S, Lefebvre D 1995 Oxytocin and oxytocin receptor gene expression in the uterus. Recent Prog Horm Res 50: 255–273.
Fuchs AR, Fuchs F 1984 Endocrinology of human parturition: a review. Br J Obstet Gynaecol 91: 948–967.
Gibbens GLD, Chard T 1976 Observations on maternal oxytocin release during human labor and the effect of intravenous alcohol administration. Am J Obstet Gynecol 126: 243–246.
Kumaresan P, Kagan A, Glick SM 1971 Oxytocin antibody and lactation and parturition in rats. Nature 230: 468–469.
Chan WY, Chen DL 1992 Myometrial oxytocin receptors and prostaglandin in the parturition process in the rat. Biol Reprod 46: 58–64.
Gross GA, Imamura T, Luedke CE, Vogt SK, Olson LM, Nelson DM, Sadovsky Y, Muglia LJ 1998 Opposing actions of prostaglandins and oxytocin determine the onset of murine labor. Proc Natl Acad Sci USA 95: 11871–11875.
Young WSI, Shepard E, Amico J, Henninghausen L, Wagner K-U, LaMarca ME, McKinney A, Ginns E 1996 Deficiency in mouse oxytocin prevents milk ejection, but not fertility or parturition. J Neuroendocrinol 8: 847–853.
Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM 1996 Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci USA 93: 11699–11704.
Kelly RW 1994 Pregnancy maintenance and parturition: the role of prostaglandin in manipulating the immune and inflammatory response. Endocr Rev 15: 684–706.
Fidel PLJ, Romero R, Wolf N, Cutright J, Ramirez M, Araneda H, Cotton DB 1994 Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol 170: 1467–1475.
Skarnes RC, Harper MJK 1972 Relationship between endotoxin-induced abortion and the synthesis of prostaglandin F. Prostaglandins 1: 191–203.
Kurki T, Eronen M, Lumme R, Ylikorkala O 1991 A randomized double-dummy comparison between indomethacin and nylidrin in threatened preterm labor. Obstet Gynecol 78: 1093–1097.
Besinger RE, Niebyl JR, Keyes WG, Johnson TRB 1991 Randomized comparative trial of indomethacin for the long-term treatment of preterm labor. Am J Obstet Gynecol 164: 981–988.
Hillier K, Calder AA, Embrey MP 1974 Concentrations of prostaglandin F2α in amniotic fluid and plasma in spontaneous and induced labours. J Obstet Gynaecol Br Commonw 81: 257–263.
Casey ML, MacDonald PC 1993 Cytokines in the human placenta, fetal membranes, uterine decidua, and amniotic fluid. In: Rice GE, Brennecke SP (eds) Molecular Aspects of Placental and Fetal Membrane Autocoids. CRC Press, Boca Raton, 361–394.
Romero R, Munoz H, Gomez R, Parra M, Polanco M, Valverde V, Hasbun J, Garrido J, Ghezzi F, Mazor M, Tolosa JE, Mitchell MD 1996 Increase in prostaglandin bioavailability precedes the onset of human parturition. Prostaglandins Leukot Essent Fatty Acids 54: 187–191.
Seibert K, Masferrer JL, Fu JY, Honda A, Raz A, Needleman P 1991 The biochemical and pharmacological manipulation of cellular cyclooxygenase (COX) activity. Adv Prostaglandin Thromboxane Leukot Res 21A: 45–51.
Flower RJ, Gryglewski R, Herbaczynska-Cedro K, Vane JR 1972 Effects of anti-inflammatory drugs on prostaglandin biosynthesis. Nature 238: 104–106.
Smith WL, Dewitt DL 1996 Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol 62: 167–215.
Simmons DL, Xie W, Chipman JG, Evett GE 1992 Multiple cyclooxygenases: cloning of a mitogen inducible form. In: Bailey JM (ed) Prostaglandins, Leukotrienes, Lipoxins, and PAF. Plenum Press, New York, 67–78.
Mijovic JE, Zakar T, Nairn TK, Olson DM 1997 Prostaglandin-endoperoxide H synthase-2 expression and activity increases with term labor in human chorion. Am J Physiol 272: E832–E840.
Evett GE, Xie W, Chipman JG, Robertson DL, Simmons DL 1993 Prostaglandin G/H synthase isoenzyme 2 expression in fibroblasts: regulation by dexamethasone, mitogens, and oncogenes. Arch Biochem Biophys 306: 169–177.
Mitchell MD, Edwin SS, Lundin-Schiller S, Silver RM, Smotkin D, Trautman MS 1993 Mechanism of interleukin-1β stimulation of human amnion prostaglandin biosynthesis: mediation via a novel inducible cyclooxygenase. Placenta 14: 615–625.
Sugimoto Y, Yamasaki A, Segi E, Tsuboi K, Aze Y, Nishimura T, Oida H, Yoshida N, Tanaka T, Katsuyama M, Hasumoto K, Murata T, Hirata M, Ushikubi F, Negishi M, Ichikawa A, Narumiya S 1997 Failure of parturition in mice lacking the prostaglandin F receptor. Science 277: 681–683.
Uozumi N, Kume K, Nagase T, Nakatani N, Ishii S, Tashiro F, Komagata Y, Maki K, Ikuta K, Ouchi Y, Miyazaki J, Shimizu T 1997 Role of cytoplasmic phospholipase A2 in allergic response and parturition. Nature 390: 618–622.
Grazzini E, Guillon G, Mouillac B, Zingg HH 1998 Inhibition of oxytocin receptor function by direct binding of progesterone. Nature 392: 509–512.
Nguyen M, Camenisch T, Snouwaert JN, Hicks E, Coffman TM, Anderson PAW, Malouf NN, Koller BH 1997 The prostaglandin receptor EP4 triggers remodelling of the cardiovascular system at birth. Nature 390: 78–81.
Tilley SL, Audoly LP, Hicks EH, Kim H-S, Flannery PJ, Coffman TM, Koller BH 1999 Reproductive failure and reduced blood pressure in mice lacking the EP2 prostaglandin E2 receptor. J Clin Invest 103: 1539–1545.
Kennedy CR, Zhang Y, Brandon S, Guan Y, Coffee K, Funk CD, Magnuson MA, Oates JA, Breyer MD, Breyer RM 1999 Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat Med 5: 217–220.
Ushikubi F, Segi E, Sugimoto Y, Murata T, Matsuoka T, Kobayashi T, Hizaki H, Tuboi K, Katsuyama M, Ichikawa A, Tanaka T, Yoshida N, Narumiya S 1998 Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature 395: 281–284.
Langenbach R, Morham SG, Tiano HF, Loftin CD, Ghanayem BI, Chulada PC, Mahler JF, Lee CA, Goulding EH, Kluckman KD, Kim HS, Smithies O 1995 Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell 83: 483–492.
Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos M, Dey SK 1997 Multiple fetal reproductive failures in cyclooxygenase-2-deficient mice. Cell 91: 197–208.
Hirst JJ, Teixeira FJ, Zakar T, Olson DM 1995 Prostaglandin H synthase-2 expression increases in human gestational tissues with spontaneous labour onset. Reprod Fertil Dev 7: 633–637.
Fuentes A, Spaziani EP, O'Brien WF 1996 The expression of cyclooxygenase-2 (COX-2) in amnion and decidua following spontaneous labor. Prostaglandins 52: 261–267.
Mijovic JE, Zakar T, Angelova J, Olson DM 1999 Prostaglandin endoperoxide H synthase mRNA expression in the human amnion and decidua during pregnancy and in the amnion at term. Mol Hum Reprod 5: 182–187.
Mijovic JE, Zakar T, Nairn TK, Olson DM 1998 Prostaglandin endoperoxide H synthase (PGHS) activity and PGHS-1 and -2 messenger ribonucleic acid abundance in human chorion throughout gestation and with preterm labor. J Clin Endocrinol Metab 83: 1358–1367.
Silver RM, Edwin SS, Trautman MS, Simmons DL, Ware Branch D, Dudley DJ, Mitchell MD 1995 Bacterial lipopolysaccharide mediated fetal death: production of a newly recognized form of inducible cyclooxygenase (COX-2) in murine decidua in response to lipopolysaccharide. J Clin Invest 95: 725–731.
Acknowledgements
I thank Drs. Joseph Majzoub, Alan Schwartz, and Jonathan Gitlin for their past and ongoing mentorship, Drs. Lauren Jacobson, Christina Luedke, Donald Bae, Gil Gross, and Takuji Imamura for their collaboration, and Sherri Vogt and Michele Schaefer for technical assistance. All mouse protocols were in accordance with National Institutes of Health guidelines and approved by the Animal Care and Use Committees of Washington University School of Medicine or Children's Hospital, Boston.
Author information
Authors and Affiliations
Additional information
Supported by grants from the National Institutes of Health, March of Dimes, Pfizer, Inc., and Monsanto Co., and a Burroughs Wellcome Fund Career Award in the Biomedical Sciences. L.J.M. is a recipient of the 1999 Society for Pediatric Research Young Investigator Award, San Francisco, CA.
Rights and permissions
About this article
Cite this article
Muglia, L. Genetic Analysis of Fetal Development and Parturition Control in the Mouse. Pediatr Res 47, 437–443 (2000). https://doi.org/10.1203/00006450-200004000-00005
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200004000-00005
This article is cited by
-
The Peter Pan paradigm
Theoretical Biology and Medical Modelling (2008)
-
Identification of 9 uterine genes that are regulated during mouse pregnancy and exhibit abnormal levels in the cyclooxygenase-1 knockout mouse
Reproductive Biology and Endocrinology (2007)
-
P-Glycoprotein and Bilirubin Disposition
Journal of Perinatology (2001)