Abstract
Children with acute lymphoblastic leukaemia (ALL) typically gain weight at excessive rates during and after therapy, and a high proportion of young adult survivors are obese. Previous studies have failed to identify the abnormalities in energy balance that predispose these children to obesity. The aim of this study was to determine the cause of excess weight gain in children treated for ALL by testing the hypothesis that energy expenditure is reduced in these patients. Twenty children [9 boys, 11 girls; mean age 10.9 (3.2) y] treated for ALL who had shown excess weight gain, but were not obese [mean body mass index SD score 0.70 (1.04)], were closely and individually matched with 20 healthy control children [9 boys, 11 girls; mean age 10.7 (3.0) y; mean body mass index SD score 0.27 (0.91)]. In each child we measured total energy expenditure by doubly-labeled water method, resting energy expenditure, energy expended on habitual physical activity, and energy intake. Total energy expenditure was significantly higher in control subjects than in patients: mean paired difference 1185 kJ/d (282 kcal/d), 95% confidence interval (CI) 218-2152. This difference was largely due to reduced energy expended on habitual physical activity in the patients. Resting energy expenditure was lower in the patients: mean paired difference 321 kJ/d (76 kcal/d), 95% CI 100-541. Energy intake was also lower in the patients: mean paired difference 1001 kJ/d (238 kcal/d), 95% CI 93-1909. Children treated for ALL are predisposed to excess weight gain, and subsequently obesity, by reduced total energy expenditure secondary to reduced habitual physical activity. Prevention of obesity in ALL should focus on modest increases in habitual physical activity, modest restriction of dietary intake, and monitoring of excess weight gain.
Similar content being viewed by others
Main
Children treated for ALL are normally nourished at diagnosis (1–3), but typically experience weight gain in excess of expected rates for at least 4 y after diagnosis, both during and after completion of chemotherapy (1–3). The prevalence of obesity in young adult survivors of ALL is approximately four times that expected (4–6), and children with ALL can be regarded as "preobese." Obesity is the result of sustained positive energy balance, but the cause of positive energy balance in ALL is unknown. Despite earlier concern over the role of cranial irradiation in the etiology of obesity in ALL (1), excess weight gain occurs even when ALL patients have not been treated with cranial radiotherapy (2,3). The use of steroids in chemotherapy may be important, but "steroid" effects such as increases in energy intake have not been observed (7), and excess weight gain occurs even after therapy has ended (1–5), implying that other factors must contribute to the problem. There is no evidence that children treated for ALL show abnormalities in REE (3,7,9,10) or diet-induced thermogenesis (10), which might arise from the effects of cranial irradiation on sympathetic nervous system activity, or from the effects of chemotherapy.
Energy requirement is a function of TEE (the sum of REE, diet-induced thermogenesis, and energy expended on physical activity), but no measurements of TEE in ALL have been reported. In this study we measured TEE, REE, energy expended on physical activity, and energy intake in a group of children treated for ALL who had shown excess weight gain, but were not obese, to determine which components of energy balance were responsible for excess weight gain. Measurements were compared with those obtained from a group of healthy control children who were closely matched to patients for the main determinants of energy expenditure, i.e. FFM, gender, maturational status, socioeconomic status, and season (to avoid the effect of seasonal differences in physical activity). The accuracy of methodology for measurement of energy intake is in question (8) and so testing hypotheses in relation to abnormalities of energy intake is problematic and best investigated indirectly, by the confirmation of or exclusion of abnormalities in energy expenditure (11). Our specific aim was therefore to test the hypothesis that the process of excess weight gain in patients with ALL is due to reduced energy expenditure.
METHODS
Subjects. We studied 20 healthy control children and 20 patients (9 boys, 11 girls) treated for ALL on UK Medical Research Council Protocol XI (1991-1997). A power calculation based on the observed rates of excess weight gain of patients treated at our center (1,3), with the assumption that this was entirely due to reduced TEE, suggested that a sample size of approximately 14 pairs of subjects would be adequate to test for a standardized difference in TEE of 0.70 with power 0.85 at the 5% significance level.
The long-term survival on this protocol is expected to be approximately 70% (12). The treatment schedule involved multiagent chemotherapy to induce remission followed by two or three blocks of intensive chemotherapy and therapy directed at the CNS (cranial radiotherapy, high dose methotrexate, or intrathecal methotrexate). None of the patients studied had been treated with cranial radiotherapy, and only 8/126 patients successfully treated in Scotland on the same treatment protocol received cranial radiotherapy (unpublished data). Patients then completed 100 wk of maintenance chemotherapy with daily 6-mercaptopurine, weekly methotrexate, and 4 weekly vincristine and prednisolone (12). Patients were excluded from the study if they: had relapsed; had pathology relevant to energy balance (e.g. Down's syndrome); had not shown excess weight gain (defined as a positive change in BMI, expressed as a SD score, from diagnosis or, if they had not shown a positive change in BMI SDS from diagnosis, a positive change in BMI SDS in the year preceding the study); were obese (defined as BMI SD score >2.0); or uncooperative. Applying these exclusions gave a possible sample of around 35-40 patients at our center from which we recruited our sample of 20.
Patients treated at our center (1,3) and the other Scottish centers (our unpublished data) gain weight at excess rates for at least 2 y after diagnosis, and excess weight gain continues for at least a further 2 y, after the end of therapy (1,3). For the patient group studied here the mean gain in BMI from time of diagnosis to time of the study, expressed as a change in SD score relative to UK reference data (13) was +0.8 (range -0.5 to 2.3). The mean time from diagnosis to the time of assessment was 2.9 (SD 1.2) years, and rates of excess weight gain were similar to those observed in other patients at our center (3), and other Scottish centers (unpublished data) studied over the same period: for 86 Scottish patients who had reached 2.0 y in first remission mean change in BMI SD score was 0.8 (SD 0.9). All patients were studied within 4.5 y of diagnosis, a period for which rates of excess weight gain are well characterized (1–3). All patients were in continuous complete remission at the time of assessment, and were clinically well (living at home, treated as outpatients). Sixteen of the 20 patients were studied after completion of therapy, 4/20 were studied during the second year of treatment. For these four, measurements were made at least 6 mo after intensive chemotherapy, and between 4 weekly treatments with vincristine and prednisolone.
A healthy control child was matched to each patient for the following characteristics: age (within 1 y); gender; socioeconomic status using a standard Scottish categorization scheme (14) of "Carstairs score" (1-7; 1 most affluent, 7 most deprived); FFM (within 1.5 kg of FFM using a prediction equation based on bioimpedance previously validated in Scottish children and adolescents) (15); maturational status according to the method of Tanner (16); season (measurements within each pair carried out less than 4 wk apart and controlled for school days and school holidays). Seventy-four healthy children were screened to identify the 20 recruited to the study as matched control subjects. Body weight (to 0.1 kg using digital electronic scales) and height (to 0.1 cm using a Harpenden stadiometer) were measured at the start and end of the 12-14-d study period. Body composition was also estimated from 18O dilution space using the values for hydration of FFM for children provided by Lohman (17). These date are provided for descriptive purposes only, because it was not possible to await the total body water measurements to match control subjects to patients. BMI (kg/m2) was calculated and expressed relative to UK reference data (13). FFM was estimated at the start and end of the study as described above (15). The study was approved by the hospital Ethics Committee and was carried out with the informed consent of children and their families.
Measurement of energy balance. REE was measured by indirect calorimetry using a ventilated hood indirect calorimeter (Datex Instrumentarium, Helsinki, Finland) after an overnight (10-12 h) fast and travel to the hospital by taxi, private car, or public transport. After a "settling in" period each child was measured for 12-15 min once a steady state had been reached. The coefficient of variation of the REE measurement under these conditions is 3% (3). REE was predicted for each child using the Schofield equation (18). Lack of compliance made the measurement of REE impossible in two of the patients and so predicted values were used in calculation of energy expended on habitual physical activity. Prediction of REE from the Schofield equations is unbiased in children with ALL (3), and the effect of any bias arising from the predictions should be limited given the fact that measurement of REE was not possible in only 2 of the 20 patients.
TEE was measured using the doubly-labeled water method (19,20). This provides an accurate measure of carbon dioxide production and TEE when combined with a food quotient calculated from dietary intake data (an estimate of the energy equivalent of carbon dioxide production). We applied two-point methodology (19) and equation A6 of Schoeller et al. (20). Three-day household measures records were used for the calculation of food quotient (21), and the mean food quotient was 0.87 (SD 0.02) with a mean energy equivalent for CO2 production of 23.7 kJ/L (SD 0.4). Mean ratio of dilution spaces (2H/18O) was 1.06 (SD 0.02). Each child received a sterilized, accurately weighed, oral dose of 1.6 mL/kg normalized oxygen-18 (10% enriched) mixed with 0.06 mL/kg 99.9% enriched deuterium oxide. Urine samples were obtained from each child before the dose was given, and then timed urine samples were collected the day after the dose and 12-14 d later. Isotopic enrichments of urine samples and diluted dose were analyzed in duplicate by isotope ratio mass spectrometry (BSIA Ltd, Brentford, UK). Energy expended on physical activity plus thermogenesis was calculated as TEE minus REE. Energy intake was estimated prospectively during the study period by 3-d household measures using 2 weekdays and 1 weekend day (22). The metabolizable energy content of food was calculated from a computerized version of standard UK tables (23).
A prospective element was included in the study. Changes in weight and height of patients were measured at 6 mo after the initial measurements. The aim of this was to assess associations between energy expenditure and subsequent excess weight gain, the latter expressed as the change in BMI SD score to 6 mo after the initial measurements (13).
Statistical analysis. Patients and control subjects were closely matched pairwise, so energy intake and expenditure were expressed on the basis of body mass or FFM, avoiding a mathematical artifact (24), and paired differences were analyzed; mean paired differences and 95% CIs for those differences are presented. Correlation was used to test for the association between self-reported energy intake and TEE, and to test for associations between body weight, FFM, and TEE.
RESULTS
Subjects. Characteristics of patients and control subjects are given in Table 1. Patients were slightly fatter than control subjects; mean paired difference -3.6% fat (SD 6.3; 95% CI -6.6 to -0.6). Table 1 confirms that patients and control subjects were well matched for the principal determinants of energy expenditure (FFM, age, gender, Tanner stage, season). These variables did not differ significantly between patients and control subjects (paired t tests). Fourteen pairs were prepubertal, five pubertal, and one postpubertal.
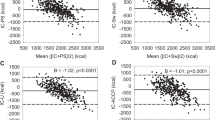
TEE. TEE was significantly higher in control subjects than in patients (Fig. 1), whether expressed on an absolute basis, mean paired difference 1185 kJ/d (282 kcal/d) 95% CI 218-2152, or when corrected for body mass, mean paired difference 44.4 kJ/kg d-1 (95% CI 20.4-68.4), or FFM, mean paired difference 40.6 kJ/kg FFM d-1 (95% CI 16.0-65.1). Both body weight (r = 0.77, p < 0.01) and FFM (r = 0.84, p < 0.01) were correlated highly with TEE.
REE. REE was slightly but significantly higher in control subjects than in patients (Fig. 1). This difference was present whether data were expressed on an absolute basis (mean paired difference 321 kJ/d-1; 95% CI 100-541) or on the basis o body mass (16.9 kJ/kg d-1, 95% CI 7.6-26.3) or FFM (mean paired difference 12.1 kJ/kg FFM d-1, 95% CI 4.4-19.7). In the patients studied, measured REE was similar to predicted values (18) (Table 1), but in the control subjects measured REE was significantly higher than predicted values (paired t test, p < 0.01; Table 1). To test this difference further, analysis of covariance (24) was used on the differences in the relationship between REE and FFM between patients and control subjects. This analysis confirmed a statistically significant difference in intercept (p < 0.05) but not in slope (p = 0.96) between the two groups.
Energy expended on physical activity. Energy expended on physical activity (plus thermogenesis) was significantly higher in control subjects than in patients (Fig. 2). The mean paired difference was 27.5 kJ/kg d-1 (95% CI 3.9-51.1), and when corrected for FFM, mean paired difference 28.5 kJ/kg FFM d-1 (95% CI 0.3-56.7).
Energy intake. Energy intake was higher in control subjects than in patients (Fig. 2), but this difference was much smaller than the difference in TEE. Mean paired difference was 1001 kJ/d-1 (95% CI 93-1909) and 39.6 kJ/kg d-1 (95% CI 4.9-74.3) when expressed on a body mass basis, 36.0 kJ/kg FFM d-1 (95% CI 0-72.0) corrected for FFM. In both patients and control subjects reported energy intakes were lower than TEE (Table 1), but this difference was not reflected in changes in body weight or composition during the study period. In both patients (r = 0.19) and control subjects (r = 0.45) energy intake was positively related to TEE, but these relationships did not reach statistical significance. The contribution of fat (36%), protein (13%), and carbohydrate (51%) to energy intake was almost identical between patient and control groups.
Prospective changes in BMI SD score. Changes in BMI SD score in the patients were not correlated with REE or energy intake, but energy expended on habitual physical activity was significantly inversely correlated with change in BMI SD score (r = -0.64, p < 0.01). Energy balance (energy intake - TEE) was positively related to change in BMI SD score in the patients but this did not reach statistical significance (r = 0.37, p > 0.05).
DISCUSSION
This study shows that the TEE of children treated for ALL is reduced relative to their healthy peers. This is the first report of abnormalities of energy balance in children treated for ALL (3,4,9,10). Because this was the first study of TEE, as distinct from REE, it emphasizes the importance of measuring the former rather than just the latter when testing hypotheses about the determinants of energy imbalance (25).
The main reason for reduced TEE in the children treated for ALL was reduced energy expended on physical activity plus thermogenesis. Diet-induced thermogenesis is a smaller and less variable component of TEE than is physical activity, and is normal in ALL (10), indicating that the difference was due to reduced habitual physical activity in the patients. It is also of note that lower energy expended on activity was associated prospectively with higher rates of excess weight gain. It is therefore reasonable to conclude that the process of excess weight gain in ALL, which is characteristic of the period of therapy and at least the first 2 y post therapy, is caused at least in part by reduced habitual physical activity. The children studied here had shown fairly typical degrees of excess weight gain for this treatment protocol (3) (unpublished data) and were broadly representative of our patients with ALL in first remission.
Reduced habitual physical activity is believed to be a risk factor for obesity (26,27), and may contribute to weight gain in patients treated for breast cancer (28), but to date there have been no well established examples of this process. Childhood ALL might therefore represent a useful model of the "preobese" state, providing valuable clues as to the mechanisms and prevention of excess weight gain. It is of note here that the patients we studied were well and relatively free from effects of therapy. Most (16/20) had completed therapy, all attended school normally, visiting the clinic every 2 wk at most, and were treated as outpatients. Under these circumstances the importance of pathologic processes that might cause excess weight gain seems limited. This strengthens the view that lifestyle is important in the etiology of the problem and that ALL might represent a useful model. An additional consideration is that ALL is not rare (400-600 new cases per year in the UK) and the children are accessible for prolonged periods during and after therapy. Models of preobesity are important in that they can provide insights into the mechanisms of dynamic weight gain, but it has been demonstrated that the existing models are limited (29). It is worth noting that we designed the study to exclude the effect of cranial radiotherapy (which is now rarely used in ALL therapy in the UK), to exclude the acute effects of intercurrent illness, and to minimize any possible acute effects of chemotherapy on energy balance. It is possible to speculate that levels of habitual activity of patients with ALL fall with hospitalization and illness during the first 2 y of therapy, and therefore that we might have underestimated the magnitude of the effect demonstrated here. It is also possible that chemotherapy might contribute to the problem by other mechanisms during the first 2 y after diagnosis.
Modest increases in habitual physical activity in children with ALL may prevent obesity. It should be possible for children with ALL to increase their level of TEE to that observed in the control children. Mean physical activity level (TEE/REE; an index of physical activity) in the patients was 1.6, whereas that of the control subjects was 1.8. We were unable to obtain data on activity patterns of the children, but the levels of habitual activity in the control group were not particularly high for children of school age (30) and imply that modest increases in activity, associated with lifestyle changes, would be adequate to change the levels of activity of the patients by a degree which would be of significance for prevention of excess weight gain. Programs aimed at increasing habitual activity, and decreasing inactivity, in children at risk of obesity can be successful (31) and are generally more effective than structured aerobic activity programs (31). For these reasons, and the potential health risk of intense aerobic activity programs for children with ALL (who might have compromised cardiopulmonary function), the focus of prevention or treatment should be on "active living" rather than on "exercise."
The difference in REE between patients and control subjects was unexpected. Our previous study of REE (3) found no evidence of such a difference. In that study we used similar design, analysis, and methods (with 27 patient-control pairs), and recruited patients who had shown excess weight gain but were not obese, as here. Three other studies of REE have failed to find abnormalities in REE in patients with ALL (7,9,10). A longitudinal study of changes in REE in patients with ALL is underway in our laboratory. A preliminary analysis of the (n = 30) patients and (n = 65) control subjects recruited to this study does not support the view that REE differs significantly between the two groups (32). In addition, variability in REE between patients at the time of the study was not significantly related to subsequent excess weight gain in the 6-mo follow-up period. Finally, the quantitatively significant difference in energy expenditure between patients and control subjects was in the energy expended on physical activity.
The study of a group of patients treated for ALL who had not shown excess weight gain might be of value, but was not possible here because of their relatively small numbers. Another potential limitation was the small but statistically significant difference in fatness between patients and control subjects. We consider this to be of minor significance to energy expenditure because of the magnitude of the difference, the relatively limited effect of fat mass on energy expenditure, and the study design that controlled for all of the major determinants of energy expenditure.
Energy intake, although lower in patients than in control subjects, did not fully compensate for reduced TEE (Fig. 2). Data on the self-reported energy intake of children must be interpreted with caution because of the problems of imprecision and inaccuracy due to underreporting (33,34). Energy expenditure data provide a more rigorous means of testing hypotheses in relation to disturbances of energy balance (8,11); there was evidence of underreporting in the present study with an apparent negative energy balance, of about 15% of TEE, in both groups (Table 1). The choice of a 3-d dietary recording period was made on practical grounds; in a pilot study compliance with the 7-d weighed intake method by the patients was poor. The mean day to day variability (coefficient of variation, %) in energy intake was 18%, which implies that for the group a 3-d period would have been adequate to estimate habitual energy intake to within 10%, but the caveats in relation to precision and accuracy at the individual level remain.
In summary, children treated for ALL who have shown excess weight gain, but are not obese, are characterized by reduced TEE relative to healthy control subjects. This difference is largely due to reduced habitual physical activity. Although this may not be the only risk factor for obesity in these children, because "steroid effects" and subtle derangements of growth hormone status might also be present (2), it is the most important factor. Future preventive strategies should concentrate on modest increases in habitual physical activity, modest restriction of dietary intake, and monitoring of excess weight gain.
Abbreviations
- ALL:
-
acute lymphoblastic leukemia
- BMI:
-
body mass index
- TEE:
-
total energy expenditure
- REE:
-
resting energy expenditure
- SDS:
-
SD score
- FFM:
-
fat-free mass
- CI:
-
confidence interval
REFERENCES
Odame I, Reilly JJ, Gibson BES, Donaldson MDC 1994 Patterns of obesity in boys and girls after treatment for ALL. Arch Dis Child 71: 147–149
Van Dongen-Melman JEWM, Hokken-Koelega ACS, Hahler K, de Groot A, Tromp CG, Egeler RM 1995 Obesity after successful treatment of acute lymphoblastic leukemia in childhood. Pediatr Res 38: 86–90
Reilly JJ, Blacklock CJ, Dale E, Donaldson MDC, Gibson BES 1996 Resting metabolic rate and obesity in childhood ALL. Int J Obes 20: 1130–1132
Didi M, Didcock E, Davies HA, Ogilvy-Stuart AL, Wales JKH, Shalet SM 1995 High incidence of obesity in young adults after treatment of ALL in childhood. J Pediatr 127: 63–67
Schell MJ, Ochs JJ, Schrock EA, Carter M 1992 A method of predicting adult height and obesity in long term survivors of childhood ALL. J Clin Oncol 10: 128–133
Zee P, Chen CH 1986 Prevalence of obesity in children after therapy for ALL. Am J Pediatr Hematol Oncol 8: 294–299
Bond SA, Han AM, Wootton SA, Kohler JA 1992 Energy intake and basal metabolic rate during maintenance chemotherapy. Arch Dis Child 67: 229–232
Sawaya AL, Tucker K, Tsay R, Willett W, Saltzman E, Dallal GE, Roberts SB 1996 Evaluation of four methods for determining energy intake in young and older women. Am J Clin Nutr 63: 491–499
Stallings VA, Vaisman N, Weitzman SS, Hahn E, Pencharz PB 1989 Energy metabolism in children with newly diagnosed acute leukemia. Pediatr Res 26: 154–157
Vaisman N, Stallings VA, Chan H, Weitzman SS, Clarke R, Pencharz PB 1993 Effect of chemotherapy on energy and protein metabolism of children near the end of treatment for ALL. Am J Clin Nutr 57: 679–684
Rosenbaum M, Ravussin E, Mathews DE, Gilker C, Ferraro R, Heymsfield SB, Hirsch J, Leibel RL 1996 A comparison of different means of assessing energy expenditure in humans. Am J Physiol 270: R496–R504
Burnett AK, Eden OB 1997 The treatment of acute leukaemia. Lancet 349: 270–275
Cole TJ, Freeman JV, Preece MA 1995 Body mass index reference curves for the UK, 1990. Arch Dis Child 73: 25–29
Carstairs V, Morris R 1991 Deprivation and Health in Scotland. Aberdeen University Press, Aberdeen, UK, 18–37.
Reilly JJ, Wilson J, McColl J, Carmichael M, Durnin JVGA 1996 Ability of bioelectric impedance to predict fat-free mass in prepubertal children. Pediatr Res 39: 176–179
Tanner JM 1962 Growth at Adolescence, 2nd Ed. Blackwell Scientific Publications, Oxford, UK. 1–94.
Lohman TG 1993 Estimating body composition in children and the elderly. Advances in Body Composition Assessment, Monograph 3. Human Kinetics Publishers, Champaign, IL, 65–79.
Schofield WN 1985 Predicting basal metabolic rate. Hum Nutr Clin Nutr 39C( suppl 1): 5-41
Welle S 1990 Two point vs. multipoint sample collection for the analysis of energy expenditure by use of doubly labelled water. Am J Clin Nutr 52: 1134–1138
Schoeller DA, Ravussin E, Schutz Y, Achenson KJ, Baertschi P, Jequier E 1986 Energy expenditure by doubly labelled water: validation in humans and proposed calculation. Am J Physiol 250: R823–R830
Black AE, Prentice AM, Coward WA 1986 Use of food quotients to predict respiratory quotients for the doubly labelled water method. Hum Nutr Clin Nutr 40C: 381–391
Gibson RS 1990 Food consumption of individuals In: Principles of Nutritional Assessment. Oxford University Press, Oxford, UK, 37–54.
Paul AA, Southgate DAT 1978 The Composition of Foods. HMSO, London, 203–297.
Ravussin E, Bogardus C 1989 Relationship of genetics, and physical fitness to daily energy expenditure and fuel utilisation. Am J Clin Nutr 49( suppl): 968–975
Macallan DC, Noble C, Baldwin C, Jebb SA, Prentice AM, Coward WA, Sawyer MB, McManus TJ, Griffin GE 1995 Energy expenditure and wasting in human immunodeficiency virus infection. N Engl J Med 333: 83–88
Prentice AM, Jebb SA 1995 Obesity in Britain: gluttony or sloth? BMJ 311: 437–439
Gortmaker SL, Must A, Sobol AM, Peterson K, Colditz GA, Dietz WH 1996 Television viewing as a cause of increasing obesity among children in the United States. Arch Pediatr Adolesc Med 150: 356–362
Demark-Wahnfried W, Hars V, Conaway MR, Havlin K, Rimer BK, McElveen G, and Winer EB 1997 Reduced rates of metabolism and decreased physical activity in breast cancer patients treated with adjuvant chemotherapy. Am J Clin Nutr 65: 1495–1501
Goran MI, Figeroa R, McGloin A, Nguyen V, Treuth MS, Nagy TR 1995 Obesity in children: recent advances in energy metabolism and body composition. Obes Res 3: 277–289
Torun B, Davies PSW, Livingstone MBE, Paolisso M, Sackett R, Spurr GB 1996 Energy requirements and dietary energy recommendations for children and adolescents 1-18 years old. Eur J Clin Nutr 50( suppl 1): 37s–81s
Epstein LH 1996 Exercise in the treatment of childhood obesity. Int J Obes 19( suppl 4): 117s–121s
Ventham JC, Reilly JJ, Ralston JM, Donaldson M, Gibson BES 1997 Energy balance in pre-obese children treated for ALL. Proc Nutr Soc 56: 316A
Champagne CM, Delany JP, Harsha DW, Bray GA 1996 Under-reporting of energy intake in biracial children verified by doubly labelled water. J Am Diet Assoc 96: 707–709
Livingstone MBE, Prentice AM, Coward WA, Strain JJ, Black AE, Davies PSW, Stewart CM, McKenna PG, Whitehead RG 1992 Validation of estimates of energy intake by weighted dietary record and diet history in children and adolescents. Am J Clin Nutr 56: 29–35
Acknowledgements
The authors acknowledge the help of the patients and control subjects and their families, and Diane Henderson and Evelyn Smith for their assistance in recruitment. We thank Professor Lawrence Weaver and the anonymous referees for their comments on the manuscript and Tom Aitchison for statistical advice.
Author information
Authors and Affiliations
Additional information
Supported by the Leukemia Research Fund. The indirect calorimeter was purchased with a grant from the Royal Society (J.J.R.).
Rights and permissions
About this article
Cite this article
Reilly, J., Ventham, J., Ralston, J. et al. Reduced Energy Expenditure in Preobese Children Treated for Acute Lymphoblastic Leukemia. Pediatr Res 44, 557–562 (1998). https://doi.org/10.1203/00006450-199810000-00015
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199810000-00015
This article is cited by
-
EXERCISE in pediatric autologous stem cell transplant patients: a randomized controlled trial protocol
BMC Cancer (2012)
-
Exercise in adult and pediatric hematological cancer survivors: an intervention review
Leukemia (2010)
-
Acute lymphoblastic leukemia and obesity: increased energy intake or decreased physical activity?
Supportive Care in Cancer (2009)
-
Intrahospital supervised exercise training: a complementary tool in the therapeutic armamentarium against childhood leukemia
Leukemia (2005)