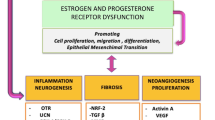
Abstract
Adenomyosis, which manifests with focally or diffusely scattered endometrial tissue within the uterine myometrium, is an endometriosis-like disease with controversial pathogenesis and compromised reproductive outcomes. This review, including the in vitro and in vivo studies performed on human or mouse models, is aimed to summarize the specific molecular characteristics of endometrium in the biochemical microenvironments of uterine adenomyosis. Many studies attributed the endometrium as the main cause of pathogenesis, with evidence of differential genetic expression and/or epigenetic modulation as well as estrogen-induced epithelial-mesenchymal transition. However, some studies indicated that the myometrium could play a role in the development of disease, based on findings of smooth muscle metaplasia and/or fibroblast-to-myofibroblast transdifferentiation by the influence of local biochemical factors. To date, it remains unclear whether adenomyosis is a genetically determined or a microenvironmentally induced disorder or whether the dysregulation of local factors may elicit the alteration of genetic expression in the endometrium. Similarly, it is uncertain whether the endometrial characteristics would remain consistent or could change along with a woman’s reproductive life. Further longitudinal studies of the epigenetic controls or system biology are needed to elucidate the pathogenesis. Discovery of effective conservative treatments to improve the reproductive outcomes of patients with adenomyosis is still warranted.
Similar content being viewed by others
References
Bird CC, McElin TW, Manalo-Estrella P. The elusive adenomyo-sis of the uterus-revisited. Am J Obstet Gynecol. 1972;112(5):583–593.
Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update. 1998;4(4):312–322.
Thain S, Tan HH. Approaches to adenomyomectomy. Gynecol Minim Invasive Ther. 2015;4(3):49–54.
Liu X, Yu S, Guo SW. A pilot study on the use of andrographolide to treat symptomatic adenomyosis. Gynecol Minim Invasive Ther. 2014;3(4):119–126.
Huang CY, Wu KY, Su H, et al. Accessibility and surgical out-comes of transumbilical single-port laparoscopy using straight instruments for hysterectomy in difficult conditions. Taiwan J Obstet Gynecol. 2014;53(4):471–475.
Han CM, Wu KY, Su H, et al. Feasibility of transumbilical single-port laparoscopic hysterectomy using conventional instruments. Gynecol Minim Invasive Ther. 2014;3(2):47–49.
Maheshwari A, Gurunath S, Fatima F, Bhattacharya S. Adeno-myosis and subfertility: a systematic review of prevalence, diagnosis, treatment and fertility outcomes. Hum Reprod Update. 2012;18(4):374–392.
Tomassetti C, Meuleman C, Timmerman D, D’Hooghe T. Ade-nomyosis and subfertility: evidence of association and causation. Semin Reprod Med. 2013;31(2):101–108.
Maubon A, Faury A, Kapella M, Pouquet M, Piver P. Uterine junctional zone at magnetic resonance imaging: a predictor of in vitro fertilization implantation failure. J Obstetrics Gynaecol Res. 2010;36(3):611–618.
Thalluri V, Tremellen KP. Ultrasound diagnosed adenomyosis has a negative impact on successful implantation following GnRH antagonist IVF treatment. Hum Reprod. 2012;27(12):3487–3492.
Vercellini P, Consonni D, Dridi D, Bracco B, Frattaruolo MP, Somigliana E. Uterine adenomyosis and in vitro fertilization out-come: a systematic review and meta-analysis. Hum Reprod. 2014;29(5):964–977.
Benagiano G, Brosens I, Carrara S. Adenomyosis: new knowledge is generating new treatment strategies. Womens Health (lond). 2009;5(3):297–311.
Leyendecker G, Herbertz M, Kunz G, Mall G. Endometriosis results from the dislocation of basal endometrium. Hum Reprod. 2002;17(10):2725–2736.
Kunz G, Beil D, Huppert P, Noe M, Kissler S, Leyendecker G. Adenomyosis in endometriosis—prevalence and impact on fertility. Evidence from magnetic resonance imaging. Hum Reprod. 2005;20(8):2309–2316.
Larsen SB, Lundorf E, Forman A, Dueholm M. Adenomyosis and junctional zone changes in patients with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2011;157(2):206–211.
Templeman C, Marshall SF, Ursin G, et al. Adenomyosis and endometriosis in the California Teachers Study. Fertil Steril. 2008;90(2):415–424.
Mechsner S, Grum B, Gericke C, Loddenkemper C, Dudenhausen JW, Ebert AD. Possible roles of oxytocin receptor and vasopressin-lalpha receptor in the pathomechanism of dysperistalsis and dysmenorrhea in patients with adenomyosis uteri. Fertil Steril. 2010;94(7):2541–2546.
Leyendecker G, Wildt L, Mall G. The pathophysiology of endometriosis and adenomyosis: tissue injury and repair. Arch Gynecol Obstet. 2009;280(4):529–538.
Barcena de Arellano ML, Gericke J, Reichelt U, et al. Immuno-histochemical characterization of endometriosis-associated smooth muscle cells in human peritoneal endometriotic lesions. Hum Reprod. 2011;26(10):2721–2730.
Hever A, Roth RB, Hevezi PA, et al. Molecular characterization of human adenomyosis. Mol Hum Reprod. 2006;12(12):737–748.
Talbi S, Hamilton AE, Vo KC, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147(3):1097–1121.
Chen YJ, Li HY, Chang YL, et al. Suppression of migratory/ invasive ability and induction of apoptosis in adenomyosisderived mesenchymal stem cells by cyclooxygenase-2 inhibitors. Fertil Steril. 2010;94(6):1972–1979, 1979.el-e4.
Jones RK, Searle RF, Bulmer JN. Apoptosis and bcl-2 expression in normal human endometrium, endometriosis and adenomyosis. Hum Reprod. 1998;13(12):3496–3502.
Yang JH, Wu MY, Chen CD, Chen MJ, Yang YS, Ho HN. Altered apoptosis and proliferation in endometrial stromal cells of women with adenomyosis. Hum Reprod. 2007;22(4):945–952.
Kim SR, Kim SH, Lee HW, Chae HD, Kim CH, Kang BM. Increased expression of p21-activated kinase in adenomyosis. Fertil Steril. 2010;94(3):1125–1128.
Yi KW, Kim SH, Ihm HJ, et al. Increased expression of p21-activated kinase 4 in adenomyosis and its regulation of matrix metalloproteinase-2 and -9 in endometrial cells. Fertil Steril. 2015;103(4):1089–1097.e2.
Yang JH, Chen MJ, Wu MY, Chen YC, Yang YS, Ho HN. Decreased suppression of interleukin-6 after treatment with medroxyprogesterone acetate and danazol in endometrial stromal cells of women with adenomyosis. Fertil Steril. 2006;86(5):1459–1465.
Wang F, Li H, Yang Z, Du X, Cui M, Wen Z. Expression of interleukin-10 in patients with adenomyosis. Fertil Steril. 2009;91(5):1681–1685.
Qin X, Zhang H, Wang F, Xue J, Wen Z. Expression and possible role of interleukin-10 receptors in patients with adenomyosis. Eur J Obstet Gynecol Reprod Biol. 2012;161(2):194–198.
Goteri G, Lucarini G, Montik N, et al. Expression of vascular endothelial growth factor (VEGF), hypoxia inducible factor-lalpha (HIF-lalpha), and microvessel density in endometrial tissue in women with adenomyosis. Int J Gynecol Pathol. 2009;28(2):157–163.
Li Y, Zou S, Xia X, Zhang S. Human adenomyosis endometrium stromal cells secreting more nerve growth factor: impact and effect. Reprod Sci. 2015;22(9):1073–1082.
Yamamoto T, Noguchi T, Tamura T, Kitawaki J, Okada H. Evidence for estrogen synthesis in adenomyotic tissues. Am J Obstet Gynecol. 1993;169(3):734–738.
Chen YJ, Li HY, Huang CH, et al. Oestrogen-induced epithelial-mesenchymal transition of endometrial epithelial cells contributes to the development of adenomyosis. J Pathol. 2010;222(3):261–270.
Nie J, Lu Y, Liu X, Guo SW. Immunoreactivity of progesterone receptor isoform B, nuclear factor kappaB, and IkappaBalpha in adenomyosis. Fertil Steril. 2009;92(3):886–889.
Mehasseb MK, Panchal R, Taylor AH, Brown L, Bell SC, Habiba M. Estrogen and progesterone receptor isoform distribution through the menstrual cycle in uteri with and without adenomyosis. Fertil Steril. 2011;95(7):2228–2235, 2235.el.
Jichan N, Xishi L, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in adenomyosis and its rectification by a histone deacetylase inhibitor and a demethylation agent. Reprod Sci. 2010;17(11):995–1005.
Liu X, Nie J, Guo SW. Elevated immunoreactivity against class I histone deacetylases in adenomyosis. Gynecol Obstet Invest. 2012;74(1):50–55.
Kang S, Zhao X, Xing H, et al. Polymorphisms in the matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 and the risk of human adenomyosis. Environ Mol Mutagen. 2008;49(3):226–231.
Kang S, Zhao J, Liu Q, Zhou R, Wang N, Li Y. Vascular endothelial growth factor gene polymorphisms are associated with the risk of developing adenomyosis. Environ Molecular Mutagen. 2009;50(5):361–366.
Kang S, Li SZ, Wang N, et al. Association between genetic polymorphisms in fibroblast growth factor (FGF)l and FGF2 and risk of endometriosis and adenomyosis in Chinese women. Hum Reprod. 2010;25(7):1806–1811.
Huang PC, Tsai EM, Li WF, et al. Association between phthalate exposure and glutathione S-transferase Ml polymorphism in adenomyosis, leiomyoma and endometriosis. Hum Reprod. 2010;25(4):986–994.
Goumenou AG, Arvanitis DA, Matalliotakis IM, Koumantakis EE, Spandidos DA. Loss of heterozygosity in adenomyosis on hMSH2, hMLH1, pl6Ink4 and GALT loci. Int J Mol Med. 2000;6(6):667–671.
Wang F, Wen Z, Li H, Yang Z, Zhao X, Yao X. Human leukocyte antigen-G is expressed by the eutopic and ectopic endometrium of adenomyosis. Fertil Steril. 2008;90(5):1599–1604.
Ren Y, Mu L, Ding X, Zheng W. Decreased expression of Beclin 1 in eutopic endometrium of women with adenomyosis. Arch Gynecol Obstet. 2010;282(4):401–406.
Liu X, Shen M, Qi Q, Zhang H, Guo SW. Corroborating evidence for platelet-induced epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis dagger. Hum Reprod. 2016;31(4):734–749.
Yang JH, Wu MY, Chang DY, Chang CH, Yang YS, Ho HN. Increased interleukin-6 messenger RNA expression in macrophage-cocultured endometrial stromal cells in adenomyosis. Am J Reprod Immunol. 2006;55(3):181–187.
Parrott E, Butterworth M, Green A, White IN, Greaves P. Adenomyosis—a result of disordered stromal differentiation. Am J Pathol. 2001;159(2):623–630.
Green AR, Styles JA, Parrott EL, et al. Neonatal tamoxifen treatment of mice leads to adenomyosis but not uterine cancer. Exp Toxicol Pathol. 2005;56(4–5):255–263.
Mehasseb MK, Bell SC, Habiba MA. The effects of tamoxifen and estradiol on myometrial differentiation and organization during early uterine development in the CD1 mouse. Reproduction. 2009;138(2):341–350.
Shen M, Liu X, Zhang H, Guo SW. Transforming growth factor betal signaling coincides with epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis in mice. Hum Reprod. 2016;31(2):355–369.
Kusakabe KT, Abe H, Kondo T, Kato K, Okada T, Otsuki Y. DNA microarray analysis in a mouse model for endometriosis and validation of candidate factors with human adenomyosis. J Reprod Immunol. 2010;85(2):149–160.
Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006;6(6):459–471.
Siu MK, Chan HY, Kong DS, et al. p21-activated kinase 4 regulates ovarian cancer cell proliferation, migration, and invasion and contributes to poor prognosis in patients. Proc Natl Acad Sci USA. 2010;107(43):18622–18627.
Vigano P, Somigliana E, Mangioni S, Vignali M, Vignali M, Di Blasio AM. Expression of interleukin-10 and its receptor is upregulated in early pregnant versus cycling human endometrium. J Clin Endocrinol Metab. 2002;87(12):5730–5736.
Verma R, Balakrishnan L, Sharma K, et al. A network map of interleukin-10 signaling pathway. J Cell Commun Signal. 2016;10(1):61–67.
Cassatella MA, Meda L, Bonora S, Ceska M, Constantin G. Interleukin 10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes. Evidence for an autocrine role of tumor necrosis factor and IL-1 beta in mediating the production of IL-8 triggered by lipopolysaccharide. J Exp Med. 1993;178(6):2207–2211.
Wang P, Wu P, Siegel MI, Egan RW, Billah MM. IL-10 inhibits transcription of cytokine genes in human peripheral blood mononuclear cells. J Immunol. 1994;153(2):811–816.
Florenzano F, Bentivoglio M. Degranulation, density, and distribution of mast cells in the rat thalamus: a light and electron microscopic study in basal conditions and after intracerebroventricular administration of nerve growth factor. J Comp Neurol. 2000;424(4):651–669.
Li Y, Zhang SF, Zou SE, Xia X, Bao L. Accumulation of nerve growth factor and its receptors in the uterus and dorsal root ganglia in a mouse model of adenomyosis. Reprod Biol Endocrinol. 2011;9:30.
Ota H, Igarashi S, Hatazawa J, Tanaka T. Is adenomyosis an immune disease? Hum Reprod Update. 1998;4(4):360–367.
Bergeron C, Amant F, Ferenczy A. Pathology and physiopathology of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20(4):511–521.
Zhou S, Yi T, Liu R, et al. Proteomics identification of annexin A2 as a key mediator in the metastasis and proangiogenesis of endometrial cells in human adenomyosis. Mol cell proteomics. 2012;11(7):M112017988.
Jaffer S, Shynlova O, Lye S. Mammalian target of rapamycin is activated in association with myometrial proliferation during pregnancy. Endocrinology. 2009;150(10):4672–4680.
Yen CF, Wang HS, Lee CL, Liao SK. Roles of integrin-linked kinase in cell signaling and its perspectives as a therapeutic target. Gynecol Minim Invasive Ther. 2014;3(3):67–72.
Tosti C, Biscione A, Morgante G, Bifulco G, Luisi S, Petraglia F. Hormonal therapy for endometriosis: from molecular research to bedside [published online May 27, 2016]. Eur J Obstet Gynecol Reprod Biol. 2016. pii: S0301-2115(16)30250-0.
Reddy BS, Rozati R, Reddy S, Kodampur S, Reddy P, Reddy R. High plasma concentrations of polychlorinated biphenyls and phthalate esters in women with endometriosis: a prospective case control study. Fertil Steril. 2006;85(3):775–779.
Duty SM, Ackerman RM, Calafat AM, Hauser R. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ Health Perspect. 2005;113(11):1530–1535.
Spurdle AB, Webb PM, Purdie DM, Chen X, Green A, Chenevix-Trench G. Polymorphisms at the glutathione S-transferase GSTM1, GSTT1 and GSTP1 loci: risk of ovarian cancer by histological subtype. Carcinogenesis. 2001;22(1):67–72.
Mechsner S, Bartley J, Loddenkemper C, Salomon DS, Starzinski-Powitz A, Ebert AD. Oxytocin receptor expression in smooth muscle cells of peritoneal endometriotic lesions and ovarian endometriotic cysts. Fertil Steril. 2005;83(suppl 1): 1220–1231.
Itoga T, Matsumoto T, Takeuchi H, et al. Fibrosis and smooth muscle metaplasia in rectovaginal endometriosis. Pathol Int. 2003;53(6):371–375.
Matsuzaki S, Darcha C. Involvement of the Wnt/beta-catenin signaling pathway in the cellular and molecular mechanisms of fibrosis in endometriosis. PIoS One. 2013;8(10):e76808.
Fukunaga M. Smooth muscle metaplasia in ovarian endometriosis. Histopathology. 2000;36(4):348–352.
Bonte H, Chapron C, Vieira M, et al. Histologic appearance of endometriosis infiltrating uterosacral ligaments in women with painful symptoms. J Am Assoc Gynecol laparosc. 2002;9(4):519–524.
van Kaam KJ, Schouten JP, Nap AW, Dunselman GA, Groothuis PG. Fibromuscular differentiation in deeply infiltrating endometriosis is a reaction of resident fibroblasts to the presence of ectopic endometrium. Hum Reprod. 2008;23(12):2692–2700.
Walter I, Handler J, Reifinger M, Aurich C. Association of endometriosis in horses with differentiation of periglandular myofibroblasts and changes of extracellular matrix proteins. Reproduction. 2001;121(4):581–586.
Riggs BL, Hartmann LC. Selective estrogen-receptor modulators—mechanisms of action and application to clinical practice. N Engl J Med. 2003;348(7):618–629.
O’Regan RM, Cisneros A, England GM, et al. Effects of the antiestrogens tamoxifen, toremifene, and ICI 182,780 on endometrial cancer growth. J Natl Cancer Inst. 1998;90(20):1552–1558.
Smas CM, Sul HS. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993;73(4):725–734.
Tanimizu N, Nishikawa M, Saito H, Tsujimura T, Miyajima A. Isolation of hepatoblasts based on the expression of Dlk/Pref-1. J Cell Set 2003;116(Pt 9):1775–1786.
Sul HS. Minireview: Pref-1: role in adipogenesis and mesenchymal cell fate. Mol Endocrinol. 2009;23(11):1717–1725.
Sloane BF. Cathepsin B and cystatins: evidence for a role in cancer progression. Semin Cancer Biol. 1990;1(2):137–152.
Ruan J, Zheng H, Rong X, et al. Over-expression of cathepsin B in hepatocellular carcinomas predicts poor prognosis of HCC patients. Mol Cancer. 2016;15:17.
Matsuzaki S, Canis M, Vaurs-Barriere C, et al. DNA microarray analysis of gene expression profiles in deep endometriosis using laser capture microdissection. Mol Hum Reprod. 2004;10(10):719–728.
Leyendecker G, Wildt L. A new concept of endometriosis and adenomyosis: tissue injury and repair (TIAR). Horm Mol Biol Clin Investig. 2011;5(2):125–142.
Khan KN, Kitajima M, Hiraki K, Fujishita A, Nakashima M, Masuzaki H. Involvement of hepatocyte growth factor-induced epithelial-mesenchymal transition in human adenomyosis. Biol Reprod. 2015;92(2):35.
Propst AM, Quade BJ, Nowak RA, Stewart EA. Granulocyte macrophage colony-stimulating factor in adenomyosis and autologous endometrium. J Soc Gynecol Investig. 2002;9(2):93–97.
Tremellen KP, Russell P. The distribution of immune cells and macrophages in the endometrium of women with recurrent reproductive failure. II: adenomyosis and macrophages. J Reprod Immunol. 2012;93(1):58–63.
Yang JH, Chen MJ, Chen HF, Lee TH, Ho HN, Yang YS. Decreased expression of killer cell inhibitory receptors on natural killer cells in eutopic endometrium in women with adenomyosis. Hum Reprod. 2004;19(9):1974–1978.
Lash GE, Robson SC, Bulmer JN. Review: Functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta. 2010;31(suppl):S87–S92.
Gui T, Chen C, Zhang Z, et al. The disturbance of TH17-Treg cell balance in adenomyosis. Fertil Steril. 2014;101(2):506–514.
Guo SW. Keep the pressure on for more transparency of clinical trials on endometriosis. Gynecol Minim Invasive Ther. 2013;2(3):73–74.
Matarese G, De Placido G, Nikas Y, Alviggi C. Pathogenesis of endometriosis: natural immunity dysfunction or autoimmune disease? Trends Mol Med. 2003;9(5):223–228.
Bulun SE, Monsivais D, Kakinuma T, et al. Molecular biology of endometriosis: from aromatase to genomic abnormalities. Semin Reprod Med. 2015;33(3):220–224.
Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398.
Wang KC, Chang WH, Lee WL, et al. An increased risk of epithelial ovarian cancer in Taiwanese women with a new surgico-pathological diagnosis of endometriosis. BMC Cancer. 2014;14:831.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yen, CF., Huang, S.J., Lee, CL. et al. Molecular Characteristics of the Endometrium in Uterine Adenomyosis and Its Biochemical Microenvironment. Reprod. Sci. 24, 1346–1361 (2017). https://doi.org/10.1177/1933719117691141
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719117691141