Abstract
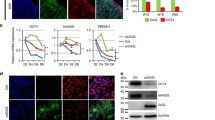
Differentiating embryonic stem cells (ESCs) can form ovarian follicle-like structures in vitro, consisting of an oocyte-like cell surrounded by somatic cells capable of steroidogenesis. Using a dual-fluorescence reporter system in which mouse ESCs express green fluorescent protein (GFP) under the control of a germ cell–specific Pou5f1 gene promoter and red fluorescent protein (Discosoma sp red [DsRed]) driven by the granulosa cell–specific Forkhead box L2 (Foxl2) gene promoter, we first confirmed in vitro formation of follicle-like structures containing GFP-positive cells surrounded by DsRed-positive cells. Isolated DsRed-positive cells specified from ECSs exhibited a gene expression profile consistent with granulosa cells, as revealed by the detection of messenger RNAs (mRNAs) for Foxl2, follistatin (Fst), anti-Müllerian hormone (Amh), and follicle-stimulating hormone receptor (Fshr) as well as by production of both progesterone and estradiol. In addition, treatment of isolated DsRed-expressing cells with follicle-stimulating hormone (FSH) significantly increased estradiol production over basal levels, confirming the presence of functional FSH receptors in these cells. Last, ESC-derived DsRed-positive cells injected into neonatal mouse ovaries became incorporated within the granulosa cell layer of immature follicles. These studies demonstrate that Foxl2-expressing ovarian somatic cells derived in vitro from differentiating ESCs express granulosa cell markers, actively associate with germ cells in vitro, synthesize steroids, respond to FSH, and participate in folliculogenesis in vivo.
Similar content being viewed by others
References
Hübner K, Fuhrmann G, Christenson LK, et al. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300(5623):1251–1256.
Hayashi K, Ogushi S, Kurimoto K, Shimamoto S, Ohta H, Saitou M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science. 2012;338(6109):971–975.
Zou K, Yuan Z, Yang Z, et al Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11(5):631–636.
Zhang Y, Yang Z, Yang Y, et al Production of transgenic mice by random recombination of targeted genes in female germline stem cells. J Mol Cell Biol. 2011;3(2):132–141.
White YAR, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18(3):413–421.
Woods DC, White YAR, Tilly JL. Purification of oogonial stem0 cells from adult mouse and human ovaries: an assessment of the literature and a view towards the future. Reprod Sci. 2013;20(1):7–15.
Nicholas CR, Haston KM, Grewall AK, Longacre TA, Reijo Pera RA. Transplantation directs oocyte maturation from embryonic stem cells and provides a therapeutic strategy for female infertility. Hum Mol Genet. 2009;18(22):4376–4389.
Nicholas CR, Chavez SL, Baker VL, Reijo Pera RA. Instructing an embryonic stem cell-derived oocyte fate: lessons from endogenous oogenesis. Endocr Rev. 2009;30(3):264–283.
Ko K, Schöler HR. Embryonic stem cells as a potential source of gametes. Semin Reprod Med. 2006;24(5):322–329.
Nagano MC. In vitro gamete derivation from pluripotent stem cells: progress and perspective. Biol Reprod. 2007;76(4):546–551.
Salvador LM, Silva CP, Kostetskii I, Radice GL, Strauss JF III. The promoter of the oocyte-specific gene, Gdf9, is active in population of cultured mouse embryonic stem cells with an oocyte-like phenotype. Methods. 2008;45(2):172–181.
Psathaki OE, Hübner K, Sabour D, et al Ultrastructural characterization of mouse embryonic stem cell-derived oocytes and granulosa cells. Stem Cells Dev. 2011;20(12):2205–2215.
Novak I, Lightfoot DA, Wang H, Eriksson A, Mahdy E, Höög C. Mouse embryonic stem cells form follicle-like ovarian structures but do not progress through meiosis. Stem Cells. 2006;24(8):1931–1936.
Albertini DF, Combelles CM, Benecchi E, Carabatsos MJ. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction. 2001;121(5):647–653.
Schmidt D, Ovitt CE, Anlag K, et al The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131(4):933–942.
Bray N, Dubchak I, Pachter L. AVID: A global alignment program. Genome Res. 2003;13(1):97–102.
Couronne O, Poliakov A, Bray N, et al Strategies and tools for whole-genome alignments. Genome Res. 2003;13(1):73–80.
Molyneaux KA, Stallock J, Schaible K, Wyllie C. Time-lapse analysis of living mouse germ cell migration. Dev Biol. 2001;240(2):488–498.
Yeom YI, Fuhrmann G, Ovitt CE, et al Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development. 1996;122(3):881–894.
Nicholas CR, Haston KM, Reijo Pera RA. Intact fetal ovarian cord formation promotes mouse oocyte survival and development. BMC Dev Biol. 2010;10:2.
Lei L, Zhang H, Jin S, et al Stage-specific germ-somatic cell interaction directs the primordial folliculogenesis in mouse fetal ovaries. J Cell Physiol. 2006;208(3):640–647.
Qing T, Liu H, Wei W, et al Mature oocytes derived from purified mouse fetal germ cells. Hum Reprod. 2008;23(1):54–61.
Hatano O, Takayama K, Imai T, et al Sex-dependent expression of a transcription factor, Ad4BP, regulating steroidogenic P-450 genes in the gonads during prenatal and postnatal rat development. Development. 1994;120(10):2787–2797.
Hatano O, Takakusu A, Nomura M, Morohashi K. Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells 1996;1(7):663–671.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Woods, D.C., White, Y.A.R., Niikura, Y. et al. Embryonic Stem Cell—Derived Granulosa Cells Participate in Ovarian Follicle Formation In Vitro and In Vivo. Reprod. Sci. 20, 524–535 (2013). https://doi.org/10.1177/1933719113483017
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719113483017