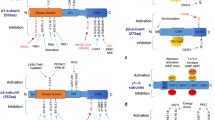
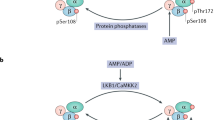
Abstract
Recently, AMP-activated protein kinase (AMPK) has emerged as a key regulator of energy balance at cellular and whole-body levels. Due to the involvement in multiple signaling pathways, AMPK efficiently controls ATP-consuming/ATP-generating processes to maintain energy homeostasis under stress conditions. Loss of the kinase activity or attenuation of its expression leads to a variety of metabolic disorders and increases cancer risk. In this review, we discuss recent findings on the structure of AMPK, its activation mechanisms, as well as the consequences of its targets in regulation of metabolism. Particular attention is given to low-molecular-weight compounds that activate or inhibit AMPK; the perspective of therapeutic use of such modulators in treatment of several common diseases is discussed.
Similar content being viewed by others
Abbreviations
- ACC:
-
acetyl-CoA carboxylase
- CaMKK:
-
Ca2+/calmodulin-dependent protein kinase kinase
- CD36:
-
cluster of differentiation 36 fatty acid translocase
- eEF2K:
-
eukaryotic elongation factor-2 kinase
- eNOS:
-
endothelial nitric oxide synthase
- FOXO3:
-
transcription factor of the forkhead family
- GLUT1/GLUT4:
-
glucose transporter 1/glucose transporter 4
- G6Pase:
-
glucose 6-phosphatase
- GPAT:
-
glycerol phosphate acyl transferase
- GS:
-
glycogen synthase
- HDAC5:
-
histone deacetylase 5
- HIF-1α:
-
hypoxia-inducible factor 1α
- HKII:
-
hexokinase II
- HMGR:
-
3-hydroxy-3-methyl-glutaryl-CoA reductase
- HSL:
-
hormone-sensitive lipase
- LKB1:
-
liver kinase B1
- MO25:
-
mouse protein-25
- mTOR:
-
mammalian target of rapamycin (kinase)
- mTORC1:
-
mTOR kinase complex 1
- NRF:
-
nuclear respiratory factor
- 6PFK2:
-
6-phosphofructo-2-kinase
- PEPCK:
-
phosphoenolpyruvate carboxykinase
- PGC-1α:
-
PPARγ coactivator 1α
- PKA:
-
protein kinase A
- PPAR:
-
peroxisome proliferator-activated receptor
- Raptor:
-
regulatory-associated protein of mTOR
- Rheb:
-
GTP-binding protein
- SREBP-1c:
-
sterol regulatory element-binding protein 1c
- STRAD:
-
STE20-related kinase adapter protein
- TAK1:
-
TGF-β-activated kinase-1
- TBC1D1/TBC1D4:
-
proteins of TBC1 domain family
- TSC2:
-
tuberous sclerosis complex 2 (tuberin)
References
Davies, S. P., Hawley S. A., Woods, A., Carling, D., Haystead, T. A., and Hardie, D. G. (1994) Purification of the AMP-activated protein kinase on ATP-gamma-Sepharose and analysis of its subunit structure, Eur. J. Biochem., 223, 351–357.
Stein, S. C., Woods, A., Jones, N. A., Davison, M. D., and Carling, D. (2000) The regulation of AMP-activated protein kinase by phosphorylation, Biochem. J., 345, 437–443.
Dyck, J. R., Gao, G., Widmer, J., Stapleton, D., Fernandez, C. S., Kemp, B. E., and Witters, L. A. (1996) Regulation of 5′-AMP-activated protein kinase activity by the noncatalytic beta and gamma subunits, J. Biol. Chem., 271, 17798–17803.
Stapleton, D., Woollatt, E., Mitchelhill, K. I., Nicholl, J. K., Fernandez, C. S., Michell, B. J., Witters, L. A., Power, D. A., Sutherland, G. R., and Kemp, B. E. (1997) AMP-activated protein kinase isoenzyme family: subunit structure and chromosomal location, FEBS Lett., 409, 452–456.
Mitchelhill, K. I., Stapleton, D., Gao, G., House, C., Michell, B., Katsis, F., Witters, L. A., and Kemp, B. E. (1994) AMP-activated protein kinase shares structural and functional homology with the catalytic domain of yeast Snf1 protein kinase, J. Biol. Chem., 269, 2361–1364.
Crute, B. E., Seefeld, K., Gamble, J., Kemp, B. E., and Witters, L. A. (1998) Functional domains of the alpha1 catalytic subunit of the AMP-activated protein kinase, J. Biol. Chem., 273, 35347–35354.
Hawley, S. A., Davison, M., Woods, A., Davies, S. P., Beri, R. K., Carling, D., and Hardie, D. G. (1996) Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase, J. Biol. Chem., 271, 27879–27887.
Littler, D. R., Walker, J. R., Davis, T., Wybenga-Groot, L. E., Finerty, P. J., Jr., Newman, E., Mackenzie, F., and Dhe-Paganon, S. (2010) A conserved mechanism of autoinhibition for the AMPK kinase domain: ATP-binding site and catalytic loop refolding as a means of regulation, Acta Crystallogr. Sec. F Struct. Biol. Cryst. Commun., 66, 143–151.
Thornton, C., Snowden, M. A., and Carling, D. (1998) Identification of a novel AMP-activated protein kinase beta subunit isoform that is highly expressed in skeletal muscle, J. Biol. Chem., 273, 12443–12450.
Xiao, B., Sanders, M. J., Underwood, E., Heath, R., Mayer, F. V., Carmena, D., Jing, C., Walker, P. A., Eccleston, J. F., Haire, L. F., Saiu, P., Howell, S. A., Aasland, R., Martin, S. R., Carling, D., and Gamblin, S. J. (2011) Structure of mammalian AMPK and its regulation by ADP, Nature, 472, 230–233.
Salt, I., Celler, J. W., Hawley, S. A., Prescott, A., Woods, A., Carling, D., and Hardie, D. G. (1998) AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the alpha2 isoform, Biochem. J., 334, 177–187.
Kazgan, N., Williams, T., Forsberg, L. J., and Brenman, J. E. (2010) Identification of a nuclear export signal in the catalytic subunit of AMP-activated protein kinase, Mol. Biol. Cell, 21, 3433–3442.
Woods, A., Cheung, P. C., Smith, F. C., Davison, M. D., Scott, J., Beri, R. K., and Carling, D. (1996) Characterization of AMP-activated protein kinase beta and gamma subunits. Assembly of the heterotrimeric complex in vitro, J. Biol. Chem., 271, 10282–10290.
Bieri, M., Mobbs, J. I., Koay, A., Louey, G., Mok, Y. F., Hatters, D. M., Park, J. T., Park, K. H., Neumann, D., Stapleton, D., and Gooley, P. R. (2012) AMP-activated protein kinase β-subunit requires internal motion for optimal carbohydrate binding, Biophys. J., 102, 305–314.
McBride, A., Ghilagaber, S., Nikolaev, A., and Hardie, D. G. (2009) The glycogen-binding domain on the AMPK beta subunit allows the kinase to act as a glycogen sensor, Cell Metab., 9, 23–34.
Iseli, T. J., Walter, M., van Denderen, B. J., Katsis, F., Witters, L. A., Kemp, B. E., Michell, B. J., and Stapleton, D. (2005) AMP-activated protein kinase beta subunit tethers alpha and gamma subunits via its C-terminal sequence (186-270), J. Biol. Chem., 280, 13395–13400.
Warden, S. M., Richardson, C., O’Donnell, J., Jr., Stapleton, D., Kemp, B. E., and Witters, L. A. (2001) Post-translational modifications of the beta-1 subunit of AMP-activated protein kinase affect enzyme activity and cellular localization, Biochem. J., 354, 275–283.
Moffat, C., and Harper, E. M. (2010) Metabolic functions of AMPK: aspects of structure and of natural mutations in the regulatory gamma subunits, IUBMB Life, 62, 739–745.
Cheung, P. C., Salt, I. P., Davies, S. P., Hardie, D. G., and Carling, D. (2000) Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding, Biochem. J., 346, 659–669.
Scott, J. W., Hawley, S. A., Green, K. A., Anis, M., Stewart, G., Scullion, G. A., Norman, D. G., and Hardie, D. G. (2004) CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations, J. Clin. Invest., 113, 274–284.
Viana, R., Towler, M. C., Pan, D. A., Carling, D., Viollet, B., Hardie, D. G., and Sanz, P. (2007) A conserved sequence immediately N-terminal to the Bateman domains in AMP-activated protein kinase gamma subunits is required for the interaction with the beta subunits, J. Biol. Chem., 282, 16117–16125.
Chen, L., Jiao, Z. H., Zheng, L. S., Zhang, Y. Y., Xie, S. T., Wang, Z. X., and Wu, J. W. (2009) Structural insight into the autoinhibition mechanism of AMP-activated protein kinase, Nature, 459, 1146–1149.
Rudolph, M. J., Amodeo, G. A., and Tong, L. (2010) An inhibited conformation for the protein kinase domain of the Saccharomyces cerevisiae AMPK homolog Snf1, Acta Crystallogr. Sec. F Struct. Biol. Cryst. Commun., 66, 999–1002.
Townley, R., and Shapiro, L. (2007) Crystal structures of the adenylate sensor from fission yeast AMP-activated protein kinase, Science, 315, 1726–1729.
Xiao, B., Heath, R., Saiu, P., Leiper, F. C., Leone, P., Jing, C., Walker, P. A., Haire, L., Eccleston, J. F., Davis, C. T., Martin, S. R., Carling, D., and Gamblin, S. J. (2007) Structural basis for AMP binding to mammalian AMP-activated protein kinase, Nature, 449, 496–500.
Mayer, F. V., Heath, R., Underwood, E., Sanders, M. J., Carmena, D., McCartney, R. R., Leiper, F. C., Xiao, B., Jing, C., Walker, P. A., Haire, L. F., Ogrodowicz, R., Martin, S. R., Schmidt, M. C., Gamblin, S. J., and Carling, D. (2011) ADP regulates SNF1, the Saccharomyces cerevisiae homolog of AMP-activated protein kinase, Cell Metab., 14, 707–714.
Amodeo, G. A., Rudolph, M. J., and Tong, L. (2007) Crystal structure of the heterotrimer core of Saccharomyces cerevisiae AMPK homologue SNF1, Nature, 449, 492–455.
Chen, L., Xin, F. J., Wang, J., Hu, J., Zhang, Y. Y., Wan, S., Cao, L. S., Lu, C., Li, P., Yan, S. F., Neumann, D., Schlattner, U., Xia, B., Wang, Z. X., and Wu, J. W. (2013) Conserved regulatory elements in AMPK, Nature, 498, E8–10.
Ghillebert, R., Swinnen, E., Wen, J., Vandesteene, L., Ramon, M., Norga, K., Rolland, F., and Winderickx, J. (2011) The AMPK/SNF1/SnRK1 fuel gauge and energy regulator: structure, function and regulation, FEBS J., 278, 3978–3990.
Hardie, D. G., and Hawley, S. A. (2001) AMP-activated protein kinase: the energy charge hypothesis revisited, Bioessays, 23, 1112–1119.
Mungai, P. T., Waypa, G. B., Jairaman, A., Prakriya, M., Dokic, D., Ball, M. K., and Schumacker, P. T. (2011) Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels, Mol. Cell. Biol., 31, 3531–3545.
Marsin, A. S., Bertrand, L., Rider, M. H., Deprez, J., Beauloye, C., Vincent, M. F., Van den Berghe, G., Carling, D., and Hue, L. (2000) Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischemia, Curr. Biol., 10, 1247–1255.
Wu, S. B., and Wei, Y. H. (2012) AMPK-mediated increase of glycolysis as an adaptive response to oxidative stress in human cells: implication of the cell survival in mitochondrial diseases, Biochim. Biophys. Acta, 1822, 233–247.
Musi, N., Fujii, N., Hirshman, M. F., Ekberg, I., Froberg, S., Ljungqvist, O., Thorell, A., and Goodyear, L. J. (2001) AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise, Diabetes, 50, 921–927.
Johnson, L. N., Noble, M. E., and Owen, D. J. (1996) Active and inactive protein kinases: structural basis for regulation, Cell, 85, 149–158.
Suter, M., Riek, U., Tuerk, R., Schlattner, U., Wallimann, T., and Neumann, D. (2006) Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase, J. Biol. Chem., 281, 32207–32216.
Hardie, D. G., Ross, F. A., and Hawley, S. A. (2012) AMP-activated protein kinase: a target for drugs both ancient and modern, Chem. Biol., 19, 1222–1236.
Viollet, B., Horman, S., Leclerc, J., Lantier, L., Foretz, M., Billaud, M., Giri, S., and Andreelli, F. (2010) AMPK inhibition in health and disease, Crit. Rev. Biochem. Mol. Biol., 45, 276–295.
Sanders, M. J., Grondin, P. O., Hegarty, B. D., Snowden, M. A., and Carling, D. (2007) Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade, Biochem. J., 403, 139–148.
Chen, L., Wang, J., Zhang, Y. Y., Yan, S. F., Neumann, D., Schlattner, U., Wang, Z. X., and Wu, J. W. (2012) AMP-activated protein kinase undergoes nucleotide-dependent conformational changes, Nat. Struct. Mol. Biol., 19, 716–719.
Oakhill, J. S., Chen, Z. P., Scott, J. W., Steel, R., Castelli, L. A., Ling, N., Macaulay, S. L., and Kemp, B. E. (2010) β-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase, Proc. Natl. Acad. Sci. USA, 107, 19237–19241.
Jin, X., Townley, R., and Shapiro, L. (2007) Structural insight into AMPK regulation: ADP comes into play, Structure, 15, 1285–1295.
Shaw, R. J., Kosmatka, M., Bardeesy, N., Hurley, R. L., Witters, L. A., DePinho, R. A., and Cantley, L. C. (2004) The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress, Proc. Natl. Acad. Sci. USA, 101, 3329–3335.
Woods, A., Dickerson, K., Heath, R., Hong, S. P., Momcilovic, M., Johnstone, S. R., Carlson, M., and Carling, D. (2005) Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells, Cell Metab., 2, 21–33.
Momcilovic, M., Hong, S. P., and Carlson, M. (2006) Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro, J. Biol. Chem., 281, 25336–25343.
Woods, A., Johnstone, S. R., Dickerson, K., Leiper, F. C., Fryer, L. G., Neumann, D., Schlattner, U., Wallimann, T., Carlson, M., and Carling, D. (2003) LKB1 is the upstream kinase in the AMP-activated protein kinase cascade, Curr. Biol., 13, 2004–2008.
Baas, A. F., Boudeau, J., Sapkota, G. P., Smit, L., Medema, R., Morrice, N. A., Alessi, D. R., and Clevers, H. C. (2003) Activation of the tumor suppressor kinase LKB1 by the STE20-like pseudokinase STRAD, EMBO J., 22, 3062–3072.
Boudeau, J., Baas, A. F., Deak, M., Morrice, N. A., Kieloch, A., Schutowski, M., Prescott, A. R., Clevers, H. C., and Alessi, D. R. (2003) MO25α/β interact with STRADα/β enhancing their ability to bind, activate and localize LKB1 in the cytoplasm, EMBO J., 22, 5102–5104.
McInnes, K. J., Brown, K. A., Hunger, N. I., and Simpson, E. R. (2012) Regulation of LKB1 expression by sex hormones in adipocytes, Int. J. Obes. (Lond.), 36, 982–985.
Brown, K. A., McInnes, K. J., Takagi, K., Ono, K., Hunger, N. I., Wang, L., Sasano, H., and Simpson, E. R. (2011) LKB1 expression is inhibited by estradiol-17β in MCF-7 cells, J. Steroid Biochem. Mol. Biol., 127, 439–443.
Marignani, P. A. (2005) LKB1, the multitasking tumor suppressor kinase, J. Clin. Pathol., 58, 15–19.
Ji, H., Ramsey, M. R., Hayes, D. N., Fan, C., McNamara, K., Kozlowski, P., Torrice, C., Wu, M. C., Shimamura, T., Perera, S. A., Liang, M. C., Cai, D., Naumov, G. N., Bao, L., Contreras, C. M., Li, D., Chen, L., Krishnamurthy, J., Koivunen, J., Chirieac, L. R., Padera, R. F., Bronson, R. T., Lindeman, N. I., Christiani, D. C., Lin, X., Shapiro, G. I., Janne, P. A., Johnson, B. E., Meyerson, M., Kwiatkowski, D. J., Castrillon, D. H., Bardeesy, N., Sharpless, N. E., and Wong, K. K. (2007) LKB1 modulates lung cancer differentiation and metastasis, Nature, 448, 807–810.
Lizcano, J. M., Goransson, O., Toth, R., Deak, M., Morrice, N. A., Boudeau, J., Hawley, S. A., Udd, L., Makela, T. P., Hardie, D. G., and Alessi, D. R. (2004) LKB1 is a master kinase that activates 13 protein kinases of the AMPK subfamily, including the MARK/PAR-1 kinases, EMBO J., 23, 833–843.
Hawley, S. A., Boudeau, J., Reid, J. L., Mustard, K. J., Udd, L., Makela, T. P., Alessi, D. R., and Hardie, D. G. (2003) Complexes between the LKB1 tumor suppressor, STRADα/β and MO25α/β are upstream kinases in the AMP-activated protein kinase cascade, J. Biol., 2, 28.
Hurley, R. L., Anderson, K. A., Franzone, J. M., Kemp, B. E., Means, A. R., and Witters, L. A. (2005) The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases, J. Biol. Chem., 280, 29060–29066.
Hawley, S. A., Pan, D. A., Mustard, K. J., Ross, L., Bain, J., Edelman, A. M., Frenguelli, B. G., and Hardie, D. G. (2005) Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase, Cell. Metab., 2, 9–19.
Stahmann, N., Woods, A., Carling, D., and Heller, R. (2006) Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase b, Mol. Cell. Biol., 26, 5933–5945.
Tamas, P., Hawley, S. A., Clarke, R. G., Mustard, K. J., Green, K., Hardie, D. G., and Cantrell, D. A. (2006) Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes, J. Exp. Med., 203, 1665–1670.
Xie, M., Zhang, D., Dyck, J. R., Li, Y., Zhang, H., Morishima, M., Mann, D. L., Taffet, G. E., Baldini, A., Khoury, D. S., and Schneider, M. D. (2006) A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway, Proc. Natl. Acad. Sci. USA, 103, 17378–17383.
Chida, T., Ando, M., Matsuki, T., Masu, Y., Nagaura, Y., Takano-Yamamoto, T., Tamura, S., and Kobayashi, T. (2013) N-Myristoylation is essential for protein phosphatases PPM1A and PPM1B to dephosphorylate their physiological substrates in cells, Biochem. J., 449, 741–749.
Steinberg, G. R., and Kemp, B. E. (2009) AMPK in health and disease, Physiol. Rev., 89, 1025–1078.
Hurley, R. L., Barre, L. K., Wood, S. D., Anderson, K. A., Kemp, B. E., Means, A. R., and Witters, L. A. (2006) Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP, J. Biol. Chem., 281, 36662–36672.
Lin, Y. Y., Kiihl, S., Suhail, Y., Liu, S. Y., Chou, Y. H., Kuang, Z., Lu, J. Y., Khor, C. N., Lin, C. L., Bader, J. S., Irizarry, R., and Boeke, J. D. (2012) Functional dissection of lysine deacetylases reveals that HDAC1 and p300 regulate AMPK, Nature, 482, 251–255.
Kola, B., Boscaro, M., Rutter, G. A., Grossman, A. B., and Korbonits, M. (2006) Expanding role of AMPK in endocrinology, Trends Endocrinol. Metab., 17, 205–215.
Stark, R., Ashley, S. E., and Andrews, Z. B. (2013) AMPK and the neuroendocrine regulation of appetite and energy expenditure, Mol. Cell. Endocrinol., 366, 215–223.
Frosig, C., Pehmoller, C., Birk, J. B., Richter, E. A., and Wojtaszewski, J. F. (2010) Exercise-induced TBC1D1 Ser237 phosphorylation and 14-3-3 protein binding capacity in human skeletal muscle, J. Physiol., 588, 4539–4548.
Barnes, K., Ingram, J. C., Porras, O. H., Barros, L. F., Hudson, E. R., Fryer, L. G., Foufelle, F., Carling, D., Hardie, D. G., and Baldwin, S. A. (2002) Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK), J. Cell. Sci., 115, 2433–2442.
McGee, S. L., van Denderen, B. J., Howlett, K. F., Mollica, J., Schertzer, J. D., Kemp, B. E., and Hargreaves, M. (2008) AMP-activated protein kinase regulates GLUT4 transcription by phosphorylating histone deacetylase 5, Diabetes, 57, 860–867.
Jorgensen, S. B., Nielsen, J. N., Birk, J. B., Olsen, G. S., Viollet, B., Andreelli, F., Schjerling, P., Vaulont, S., Hardie, D. G., Hansen, B. F., Richter, E. A., and Wojtaszewski, J. F. (2004) The alpha2-5′AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading, Diabetes, 53, 3074–3081.
Marsin, A. S., Bouzin, C., Bertrand, L., and Hue, L. (2002) The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase, J. Biol. Chem., 277, 30778–30783.
Lochhead, P. A., Salt, I. P., Walker, K. S., Hardie, D. G., and Sutherland, C. (2000) 5-Aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase, Diabetes, 49, 896–903.
Davies, S. P., Carling, D., Munday, M. R., and Hardie, D. G. (1992) Diurnal rhythm of phosphorylation of rat liver acetyl-CoA carboxylase by the AMP-activated protein kinase, demonstrated using freeze-clamping. Effects of high fat diets, Eur. J. Biochem., 203, 615–623.
Li, Y., Xu, S., Mihaylova, M. M., Zheng, B., Hou, X., Jiang, B., Park, O., Luo, Z., Lefai, E., Shyy, J. Y., Gao, B., Wierzbicki, M., Verbeuren, T. J., Shaw, R. J., Cohen, R. A., and Zang, M. (2011) AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice, Cell. Metab., 13, 376–388.
Merrill, G. F., Kurth, E. J., Hardie, D. G., and Winder, W. W. (1997) AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle, Am. J. Physiol., 273, E1107–1112.
Habets, D. D., Coumans, W. A., El Hasnaoui, M., Zarrinpashneh, E., Bertrand, L., Viollet, B., Kiens, B., Jensen, T. E., Richter, E. A., Bonen, A., Glatz, J. F., and Luiken, J. J. (2009) Crucial role for LKB1 to AMPKalpha2 axis in the regulation of CD36-mediated long-chain fatty acid uptake into cardiomyocytes, Biochim. Biophys. Acta, 1791, 212–219.
Daval, M., Diot-Dupuy, F., Bazin, R., Hainault, I., Viollet, B., Vaulont, S., Hajduch, E., Ferre, P., and Foufelle, F. (2005) Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes, J. Biol. Chem., 280, 25250–25257.
Clarke, P. R., and Hardie, D. G. (1990) Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver, EMBO J., 9, 2439–2446.
Muoio, D. M., Seefeld, K., Witters, L. A., and Coleman, R. A. (1999) AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target, Biochem. J., 338, 783–791.
Inoki, K., Zhu, T., and Guan, K. L. (2003) TSC2 mediates cellular energy response to control cell growth and survival, Cell, 115, 577–590.
Browne, G. J., Finn, S. G., and Proud, C. G. (2004) Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398, J. Biol. Chem., 279, 12220–12231.
Jones, R. G., Plas, D. R., Kubek, S., Buzzai, M., Mu, J., Xu, Y., Birnbaum, M. J., and Thompson, C. B. (2005) AMP-activated protein kinase induces a p53-dependent metabolic checkpoint, Mol. Cell, 18, 283–293.
Liang, J., Shao, S. H., Xu, Z. X., Hennessy, B., Ding, Z., Larrea, M., Kondo, S., Dumont, D. J., Gutterman, J. U., Walker, C. L., Slingerland, J. M., and Mills, G. B. (2007) The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis, Nat. Cell. Biol., 9, 218–224.
Greer, E. L., Oskoui, P. R., Banko, M. R., Maniar, J. M., Gygi, M. P., Gygi, S. P., and Brunet, A. (2007) The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor, J. Biol. Chem., 282, 30107–30119.
Procopio, C., Andreozzi, F., Laratta, E., Cassese, A., Beguinot, F., Arturi, F., Hribal, M. L., Perticone, F., and Sesti, G. (2009) Leptin-stimulated endothelial nitric-oxide synthase via an adenosine 5′-monophosphate-activated protein kinase/Akt signaling pathway is attenuated by interaction with C-reactive protein, Endocrinology, 150, 3584–3593.
Lin, J., Handschin, C., and Spiegelman, B. M. (2005) Metabolic control through the PGC-1 family of transcription coactivators, Cell. Metab., 1, 361–370.
Stapleton, D., Mitchelhill, K. I., Gao, G., Widmer, J., Michell, B. J., Teh, T., House, C. M., Fernandez, C. S., Cox, T., Witters, L. A., and Kemp, B. E. (1996) Mammalian AMP-activated protein kinase subfamily, J. Biol. Chem., 271, 611–614.
Kim, M., and Tian, R. (2011) Targeting AMPK for cardiac protection: opportunities and challenges, J. Mol. Cell. Cardiol., 51, 548–553.
Zhou, G., Myers, R., Li, Y., Chen, Y., Shen, X., Fenyk-Melody, J., Wu, M., Ventre, J., Doebber, T., Fujii, N., Musi, N., Hirshman, M. F., Goodyear, L. J., and Moller, D. E. (2001) Role of AMP-activated protein kinase in mechanism of metformin action, J. Clin. Invest., 108, 1167–1174.
Breen, D. M., Sanli, T., Giacca, A., and Tsiani, E. (2008) Stimulation of muscle cell glucose uptake by resveratrol through sirtuins and AMPK, Biochem. Biophys. Res. Commun., 374, 117–122.
Lee, Y. S., Kim, W. S., Kim, K. H., Yoon, M. J., Cho, H. J., Shen, Y., Ye, J. M., Lee, C. H., Oh, W. K., Kim, C. T., Hohnen-Behrens, C., Gosby, A., Kraegen, E. W., James, D. E., and Kim, J. B. (2006) Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states, Diabetes, 55, 2256–2264.
Li, H. B., Ge, Y. K., Zheng, X. X., and Zhang, L. (2008) Salidroside stimulated glucose uptake in skeletal muscle cells by activating AMP-activated protein kinase, Eur. J. Pharmacol., 588, 165–169.
Gruzman, A., Shamni, O., Ben Yakir, M., Sandovski, D., Elgart, A., Alpert, E., Cohen, G., Hoffman, A., Katzhendler, Y., Cerasi, E., and Sasson, S. (2008) Novel D-xylose derivatives stimulate muscle glucose uptake by activating AMP-activated protein kinase alpha, J. Med. Chem., 51, 8096–8108.
Ahn, J., Lee, H., Kim, S., Park, J., and Ha, T. (2008) The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways, Biochem. Biophys. Res. Commun., 373, 545–549.
Hwang, J. T., Park, I. J., Shin, J. I., Lee, Y. K., Lee, S. K., Baik, H. W., Ha, J., and Park, O. J. (2005) Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP-activated protein kinase, Biochem. Biophys. Res. Commun., 338, 694–699.
Kim, T., Davis, J., Zhang, A. J., He, X., and Mathews, S. T. (2009) Curcumin activates AMPK and suppresses gluconeogenic gene expression in hepatoma cells, Biochem. Biophys. Res. Commun., 388, 377–382.
Kulkarni, S. S., Karlsson, H. K., Szekeres, F., Chibalin, A. V., Krook, A., and Zierath, J. R. (2011) Suppression of 5′-nucleotidase enzymes promotes AMP-activated protein kinase (AMPK) phosphorylation and metabolism in human and mouse skeletal muscle, J. Biol. Chem., 286, 34567–34574.
Hawley, S. A., Ross, F. A., Chevtzoff, C., Green, K. A., Evans, A., Fogarty, S., Towler, M. C., Brown, L. J., Ogunbayo, O. A., Evans, A. M., and Hardie, D. G. (2010) Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation, Cell Metab., 11, 554–565.
Gruzman, A., Babai, G., and Sasson, S. (2009) Adenosine monophosphate-activated protein kinase (AMPK) as a new target for antidiabetic drugs: a review on metabolic, pharmacological and chemical considerations, Rev. Diabet. Stud., 6, 13–36.
Lehmann, J. M., Moore, L. B., Smith-Oliver, T. A., Wilkison, W. O., Willson, T. M., and Kliewer, S. A. (1995) An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma), J. Biol. Chem., 270, 12953–12956.
Fryer, L. G., Parbu-Patel, A., and Carling, D. (2002) The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways, J. Biol. Chem., 277, 25226–25232.
Krentz, A. J., Bailey, C. J., and Melander, A. (2000) Thiazolidinediones for type 2 diabetes. New agents reduce insulin resistance but need long term clinical trials, BMJ, 321, 252–253.
Corton, J. M., Gillespie, J. G., Hawley, S. A., and Hardie, D. G. (1995) 5-Aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells, Eur. J. Biochem., 229, 558–565.
Lihn, A. S., Pedersen, S. B., Lund, S., and Richelsen, B. (2008) The anti-diabetic AMPK activator AICAR reduces IL-6 and IL-8 in human adipose tissue and skeletal muscle cells, Mol. Cell. Endocrinol., 292, 36–41.
Narkar, V. A., Downes, M., Yu, R. T., Embler, E., Wang, Y. X., Banayo, E., Mihaylova, M. M., Nelson, M. C., Zou, Y., Juguilon, H., Kang, H., Shaw, R. J., and Evans, R. M. (2008) AMPK and PPARdelta agonists are exercise mimetics, Cell, 134, 405–415.
Guigas, B., Sakamoto, K., Taleux, N., Reyna, S. M., Musi, N., Viollet, B., and Hue, L. (2009) Beyond AICA riboside: in search of new specific AMP-activated protein kinase activators, IUBMB Life, 61, 18–26.
Cool, B., Zinker, B., Chiou, W., Kifle, L., Cao, N., Perham, M., Dickinson, R., Adler, A., Gagne, G., Iyengar, R., Zhao, G., Marsh, K., Kym, P., Jung, P., Camp, H. S., and Frevert, E. (2006) Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome, Cell Metab., 3, 403–416.
Goransson, O., McBride, A., Hawley, S. A., Ross, F. A., Shpiro, N., Foretz, M., Viollet, B., Hardie, D. G., and Sakamoto, K. (2007) Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase, J. Biol. Chem., 282, 32549–32560.
Scott, J. W., van Denderen, B. J., Jorgensen, S. B., Honeyman, J. E., Steinberg, G. R., Oakhill, J. S., Iseli, T. J., Koay, A., Gooley, P. R., Stapleton, D., and Kemp, B. E. (2008) Thienopyridone drugs are selective activators of AMP-activated protein kinase beta1-containing complexes, Chem. Biol., 15, 1220–1230.
Mirguet, O., Sautet, S., Clement, C. A., Toum, J., Donche, F., Marques, C., Rondet, E., Pizzonero, M., Beaufils, B., Dudit, Y., Huet, P., Trottet, L., Grondin, P., Brusq, J. M., Boursier, E., Saintillan, Y., and Nicodeme, E. (2013) Discovery of pyridones as oral AMPK direct activators, ACS Med. Chem. Lett., 4, 632–636.
Choi, J., He, N., Sung, M. K., Yang, Y., and Yoon, S. (2011) Sanguinarine is an allosteric activator of AMP-activated protein kinase, Biochem. Biophys. Res. Commun., 413, 259–263.
Scott, J. W., Ross, F. A., Liu, J. K., and Hardie, D. G. (2007) Regulation of AMP-activated protein kinase by a pseudosubstrate sequence on the gamma subunit, EMBO J., 26, 806–815.
Pang, T., Xiong, B., Li, J. Y., Qiu, B. Y., Jin, G. Z., Shen, J. K., and Li, J. (2007) Conserved alpha-helix acts as autoinhibitory sequence in AMP-activated protein kinase alpha subunits, J. Biol. Chem., 282, 495–506.
Peng, C., and Head-Gordon, T. (2011) The dynamical mechanism of auto-inhibition of AMP-activated protein kinase, PLoS Comput. Biol., 7, e1002082.
Xiao, B., Sanders, M. J., Carmena, D., Bright, N. J., Haire, L. F., Underwood, E., Patel, B. R., Heath, R. B., Walker, P. A., Hallen, S., Giordanetto, F., Martin, S. R., Carling, D., and Gamblin, S. J. (2013) Structural basis of AMPK regulation by small molecule activators, Nat. Commun., 4, 3017.
Zhu, L., Chen, L., Zhou, X. M., Zhang, Y. Y., Zhang, Y. J., Zhao, J., Ji, S. R., Wu, J. W., and Wu, Y. (2011) Structural insights into the architecture and allostery of full-length AMP-activated protein kinase, Structure, 19, 515–522.
Pang, T., Zhang, Z. S., Gu, M., Qiu, B. Y., Yu, L. F., Cao, P. R., Shao, W., Su, M. B., Li, J. Y., Nan, F. J., and Li, J. (2008) Small molecule antagonizes autoinhibition and activates AMP-activated protein kinase in cells, J. Biol. Chem., 283, 16051–16060.
Yu, L. F., Li, Y. Y., Su, M. B., Zhang, M., Zhang, W., Zhang, L. N., Pang, T., Zhang, R. T., Liu, B., Li, J. Y., Li, J., and Nan, F. J. (2013) Development of novel alkene oxindole derivatives as orally efficacious AMP-activated protein kinase activators, ACS Med. Chem. Lett., 4, 475–480.
Meltzer-Mats, E., Babai-Shani, G., Pasternak, L., Uritsky, N., Getter, T., Viskind, O., Eckel, J., Cerasi, E., Senderowitz, H., Sasson, S., and Gruzman, A. (2013) Synthesis and mechanism of hypoglycemic activity of benzothiazole derivatives, J. Med. Chem., 56, 5335–5350.
Kelley, D. E., Goodpaster, B. H., and Storlien, L. (2002) Muscle triglyceride and insulin resistance, Annu. Rev. Nutr., 22, 325–346.
Lowell, B. B., and Shulman, G. I. (2005) Mitochondrial dysfunction and type 2 diabetes, Science, 307, 384–387.
Kraegen, E. W., Saha, A. K., Preston, E., Wilks, D., Hoy, A. J., Cooney, G. J., and Ruderman, N. B. (2006) Increased malonyl-CoA and diacylglycerol content and reduced AMPK activity accompany insulin resistance induced by glucose infusion in muscle and liver of rats, Am. J. Physiol. Endocrinol. Metab., 290, E471–479.
Lee, M. J., Feliers, D., Mariappan, M. M., Sataranatarajan, K., Mahimainathan, L., Musi, N., Foretz, M., Viollet, B., Weinberg, J. M., Choudhury, G. G., and Kasinath, B. S. (2007) A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy, Am. J. Physiol. Endocrinol. Metab., 292, F617–627.
Russell, R. R., 3rd, Li, J., Coven, D. L., Pypaert, M., Zechner, C., Palmeri, M., Giordano, F. J., Mu, J., Birnbaum, M. J., and Young, L. H. (2004) AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury, J. Clin. Invest., 114, 495–503.
Calvert, J. W., Gundewar, S., Jha, S., Greer, J. J., Bestermann, W. H., Tian, R., and Lefer, D. J. (2008) Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling, Diabetes, 57, 696–705.
Kim, A. S., Miller, E. J., Wright, T. M., Li, J., Qi, D., Atsina, K., Zaha, V., Sakamoto, K., and Young, L. H. (2011) A small molecule AMPK activator protects the heart against ischemia-reperfusion injury, J. Mol. Cell. Cardiol., 51, 24–32.
Chan, A. Y., Soltys, C. L., Young, M. E., Proud, C. G., and Dyck, J. R. (2004) Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte, J. Biol. Chem., 279, 32771–32779.
Li, H. L., Yin, R., Chen, D., Liu, D., Wang, D., Yang, Q., and Dong, Y. G. (2007) Long-term activation of adenosine monophosphate-activated protein kinase attenuates pressure-overload-induced cardiac hypertrophy, J. Cell. Biochem., 100, 1086–1099.
Zhang, P., Hu, X., Xu, X., Fassett, J., Zhu, G., Viollet, B., Xu, W., Wiczer, B., Bernlohr, D. A., Bache, R. J., and Chen, Y. (2008) AMP activated protein kinase-alpha2 deficiency exacerbates pressure-overload-induced left ventricular hypertrophy and dysfunction in mice, Hypertension, 52, 918–924.
Sasaki, H., Asanuma, H., Fujita, M., Takahama, H., Wakeno, M., Ito, S., Ogai, A., Asakura, M., Kim, J., Minamino, T., Takashima, S., Sanada, S., Sugimachi, M., Komamura, K., Mochizuki, N., and Kitakaze, M. (2009) Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase, Circulation, 119, 2568–2577.
Aguilar, D., Chan, W., Bozkurt, B., Ramasubbu, K., and Deswal, A. (2011) Metformin use and mortality in ambulatory patients with diabetes and heart failure, Circ. Heart Fail., 4, 53–58.
Gundewar, S., Calvert, J. W., Jha, S., Toedt-Pingel, I., Ji, S. Y., Nunez, D., Ramachandran, A., Anaya-Cisneros, M., Tian, R., and Lefer, D. J. (2009) Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure, Circ. Res., 104, 403–411.
Ronnett, G. V., Ramamurthy, S., Kleman, A. M., Landree, L. E., and Aja, S. (2009) AMPK in the brain: its roles in energy balance and neuroprotection, J. Neurochem., 109, 17–23.
McCullough, L. D., Zeng, Z., Li, H., Landree, L. E., McFadden, J., and Ronnett, G. V. (2005) Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke, J. Biol. Chem., 280, 20493–20502.
Li, J., Zeng, Z., Viollet, B., Ronnett, G. V., and McCullough, L. D. (2007) Neuroprotective effects of adenosine monophosphate-activated protein kinase inhibition and gene deletion in stroke, Stroke, 38, 2992–2999.
Li, J., Benashski, S. E., Venna, V. R., and McCullough, L. D. (2010) Effects of metformin in experimental stroke, Stroke, 41, 2645–2652.
Ashabi, G., Khodagholi, F., Khalaj, L., Goudarzvand, M., and Nasiri, M. (2014) Activation of AMP-activated protein kinase by metformin protects against global cerebral ischemia in male rats: interference of AMPK/PGC-1α pathway, Metab. Brain Dis., 29, 47–58.
Venna, V. R., Li, J., Hammond, M. D., Mancini, N. S., and McCullough, L. D. (2014) Chronic metformin treatment improves post-stroke angiogenesis and recovery after experimental stroke, Eur. J. Neurosci., 39, 2129–2138.
Tomkins, A. M., Jones, R., and Bloom, A. (1972) Lactic acidosis occurring during phenformin therapy, Postgrad. Med. J., 48, 386–387.
Owen, M. R., Doran, E., and Halestrap, A. P. (2000) Evidence that metformin exerts its anti-diabetic effects through inhibition of complex I of the mitochondrial respiratory chain, Biochem. J., 348, 607–614.
El-Mir, M. Y., Nogueira, V., Fontaine, E., Averet, N., Rigoulet, M., and Leverve, X. (2000) Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I, J. Biol. Chem., 275, 223–228.
Zhang, Y., Wang, Y., Bao, C., Xu, Y., Shen, H., Chen, J., Yan, J., and Chen, Y. (2012) Metformin interacts with AMPK through binding to γ subunit, Mol. Cell. Biochem., 368, 69–76.
Foretz, M., Hebrard, S., Leclerc, J., Zarrinpashneh, E., Soty, M., Mithieux, G., Sakamoto, K., Andreelli, F., and Viollet, B. (2010) Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state, J. Clin. Invest., 120, 2355–2369.
Miller, R. A., Chu, Q., Xie, J., Foretz, M., Viollet, B., and Birnbaum, M. J. (2013) Biguanides suppress hepatic glucagon signaling by decreasing production of cyclic AMP, Nature, 494, 256–260.
Evans, J. M., Donnelly, L. A., Emslie-Smith, A. M., Alessi, D. R., and Morris, A. D. (2005) Metformin and reduced risk of cancer in diabetic patients, BMJ, 330, 1304–1305.
Currie, C. J., Poole, C. D., and Gale, E. A. (2009) The influence of glucose-lowering therapies on cancer risk in type 2 diabetes, Diabetologia, 52, 1766–1777.
Giovannucci, E., Harlan, D. M., Archer, M. C., Bergenstal, R. M., Gapstur, S. M., Habel, L. A., Pollak, M., Regensteiner, J. G., and Yee, D. (2010) Diabetes and cancer: a consensus report, Diabetes Care, 33, 1674–1685.
Wahdan-Alaswad, R., Fan, Z., Edgerton, S. M., Liu, B., Deng, X. S., Arnadottir, S. S., Richer, J. K., Anderson, S. M., and Thor, A. D. (2013) Glucose promotes breast cancer aggression and reduces metformin efficacy, Cell Cycle, 12, 3759–3769.
Hardie, D. G. (2013) AMPK: a target for drugs and natural products with effects on both diabetes and cancer, Diabetes, 62, 2164–2172.
Hardie, D. G., and Alessi, D. R. (2013) LKB1 and AMPK and the cancer-metabolism linkten years after, BMC Biol., 11, 36.
Faubert, B., Boily, G., Izreig, S., Griss, T., Samborska, B., Dong, Z., Dupuy, F., Chambers, C., Fuerth, B. J., Viollet, B., Mamer, O. A., Avizonis, D., DeBerardinis, R. J., Siegel, P. M., and Jones, R. G. (2013) AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo, Cell Metab., 17, 113–124.
Hsu, C. C., Wang, C. H., Wu, L. C., Hsia, C. Y., Chi, C. W., Yin, P. H., Chang, C. J., Sung, M. T., Wei, Y. H., Lu, S. H., and Lee, H. C. (2013) Mitochondrial dysfunction represses HIF-1α protein synthesis through AMPK activation in human hepatoma HepG2 cells, Biochim. Biophys. Acta, 1830, 4743–4751.
Vander Heiden, M. G., Cantley, L. C., and Thompson, C. B. (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation, Science, 324, 1029–1033.
Li, J., Benashski, S. E., Siegel, C., Liu, F., and McCullough, L. D. (2010) Adenosine monophosphate activated protein kinase inhibition is protective in both sexes after experimental stroke, Neurosci. Lett., 482, 62–65.
Vingtdeux, V., Davies, P., Dickson, D. W., and Marambaud, P. (2011) AMPK is abnormally activated in tangle- and pre-tangle-bearing neurons in Alzheimer’s disease and other tauopathies, Acta Neuropathol., 121, 337–349.
Kim, T. W., Cho, H. M., Choi, S. Y., Suguira, Y., Hayasaka, T., Setou, M., Koh, H. C., Hwang, E. M., Park, J. Y., Kang, S. J., Kim, H. S., Kim, H., and Sun, W. (2013) (ADP-ribose) polymerase 1 and AMP-activated protein kinase mediate progressive dopaminergic neuronal degeneration in a mouse model of Parkinson’s disease, Cell Death Dis., 4, e919.
Jiang, P., Gan, M., Ebrahim, A. S., Castanedes-Casey, M., Dickson, D. W., and Yen, S. H. (2013) Adenosine monophosphate-activated protein kinase overactivation leads to accumulation of α-synuclein oligomers and decrease of neurites, Neurobiol. Aging, 34, 1504–1515.
Fryer, L. G., Parbu-Patel, A., and Carling, D. (2002) Protein kinase inhibitors block the stimulation of the AMP-activated protein kinase by 5-amino-4-imidazolecarboxamide riboside, FEBS Lett., 531, 189–192.
Labuzek, K., Liber, S., Gabryel, B., Buldak, L., and Okopien, B. (2010) Ambivalent effects of compound C (dorsomorphin) on inflammatory response in LPS-stimulated rat primary microglial cultures, Naunyn-Schmiedeberg’s Arch. Pharmacol., 381, 41–57.
Bain, J., Plater, L., Elliott, M., Shpiro, N., Hastie, C. J., McLauchlan, H., Klevernic, I., Arthur, J. S., Alessi, D. R., and Cohen, P. (2007) The selectivity of protein kinase inhibitors: a further update, Biochem. J., 408, 297–315.
Handa, N., Takagi, T., Saijo, S., Kishishita, S., Takaya, D., Toyama, M., Terada, T., Shirouzu, M., Suzuki, A., Lee, S., Yamauchi, T., Okada-Iwabu, M., Iwabu, M., Kadowaki, T., Minokoshi, Y., and Yokoyama, S. (2011) Structural basis for compound C inhibition of the human AMP-activated protein kinase α2 subunit kinase domain, Acta Crystallogr. D Biol. Crystallogr., 67, 480–487.
Massillon, D., Stalmans, W., van de Werve, G., and Bollen, M. (1994) Identification of the glycogenic compound 5-iodotubercidin as a general protein kinase inhibitor, Biochem. J., 299, 123–128.
Henin, N., Vincent, M. F., and Van den Berghe, G. (1996) Stimulation of rat liver AMP-activated protein kinase by AMP analogues, Biochim. Biophys. Acta, 1290, 197–203.
Musi, N., Hayashi, T., Fujii, N., Hirshman, M. F., Witters, L. A., and Goodyear, L. J. (2001) AMP-activated protein kinase activity and glucose uptake in rat skeletal muscle, Am. J. Physiol. Endocrinol. Metab., 280, E677–684.
Kerkela, R., Woulfe, K. C., Durand, J. B., Vagnozzi, R., Kramer, D., Chu, T. F., Beahm, C., Chen, M. H., and Force, T. (2009) Sunitinib-induced cardiotoxicity is mediated by off-target inhibition of AMP-activated protein kinase, Clin. Transl. Sci., 2, 15–25.
Laderoute, K. R., Calaoagan, J. M., Madrid, P. B., Klon, A. E., and Ehrlich, P. J. (2010) SU11248 (sunitinib) directly inhibits the activity of mammalian 5′-AMP-activated protein kinase (AMPK), Cancer Biol. Ther., 10, 68–76.
Gan, H. K., Seruga, B., and Knox, J. J. (2009) Sunitinib in solid tumors, Expert. Opin. Investig. Drugs, 18, 821–834.
Machrouhi, F., Ouhamou, N., Laderoute, K., Calaoagan, J., Bukhtiyarova, M., Ehrlich, P. J., and Klona, A. E. (2010) The rational design of a novel potent analogue of the 5′-AMP-activated protein kinase inhibitor compound C with improved selectivity and cellular activity, Bioorg. Med. Chem. Lett., 20, 6394–6399.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Biokhimiya, 2015, Vol. 80, No. 2, pp. 163–183.
Rights and permissions
About this article
Cite this article
Novikova, D.S., Garabadzhiu, A.V., Melino, G. et al. AMP-activated protein kinase: Structure, function, and role in pathological processes. Biochemistry Moscow 80, 127–144 (2015). https://doi.org/10.1134/S0006297915020017
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297915020017