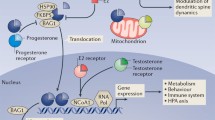
Abstract
Exposure to stress activates the hypothalamic–pituitary–adrenal axis and leads to increased levels of glucocorticoid (GC) hormones. Prolonged elevation of GC levels causes neuronal dysfunction, decreases the density of synapses, and impairs neuronal plasticity. Decreased sensitivity to glucocorticoids (glucocorticoid resistance) that develops as a result of chronic stress is one of the characteristic features of stress-induced psychopathologies. In this article, we reviewed the published data on proposed molecular mechanisms that contribute to the development of glucocorticoid resistance in brain, including changes in the expression of the glucocorticoid receptor (GR) gene, biosynthesis of GR isoforms, and GR posttranslational modifications. We also present data on alterations in the expression of the FKBP5 gene encoding the main component of cell ultra-short negative feedback loop of GC signaling regulation. Recent discoveries on stressand GRinduced changes in epigenetic modification patterns as well as normalizing action of antidepressants are discussed. GR and FKBP5 gene polymorphisms associated with stress-induced psychopathologies are described, and their role in glucocorticoid resistance is discussed.
Similar content being viewed by others
Abbreviations
- ACTH:
-
adrenocorticotropic hormone (corticotropin)
- DNMT:
-
DNA methyltransferase
- FKBP4:
-
FK506 binding protein 4 (immunophilin)
- FKBP5:
-
FK506 binding protein 5 (immunophilin)
- GC:
-
glucocorticoid hormones (glucocorticoids)
- GR:
-
glucocorticoid receptors
- GRE:
-
glucocorticoid-responsive element
- HPA:
-
hypothalamic–pituitary–adrenal axis
- IL1:
-
interleukin 1
- TNF:
-
tumor necrosis factor
- UTR:
-
untranslated region
References
Pittenger, C., and Duman, R. S. (2008) Stress, depression, and neuroplasticity: a convergence of mechanisms, Neuropsychopharmacology, 33, 88–109.
Miklowitz, D. J. (2011) Functional impairment, stress, and psychosocial intervention in bipolar disorder, Curr. Psychiatry Rep., 13, 504–512.
McEwen, B. S. (2007) Physiology and neurobiology of stress and adaptation: central role of the brain, Physiol. Rev., 87, 873–904.
Kumar, R., and Thompson, E. B. (2005) Gene regulation by the glucocorticoid receptor: structure-function relationship, J. Steroid Biochem. Mol. Biol., 94, 383–394.
Merkulov, V. M., and Merkulova, T. I. (2009) Structural variants of glucocorticoid receptor binding sites and different versions of positive glucocorticoid responsive elements: analysis of GR-TRRD database, J. Steroid Biochem. Mol. Biol., 115, 1–8.
Meijsing, S. H. (2015) Mechanisms of glucocorticoid-regulated gene transcription, Adv. Exp. Med. Biol., 872, 59–81.
Cattaneo, A., and Riva, M. A. (2016) Stress-induced mechanisms in mental illness: a role for glucocorticoid signalling, J. Steroid Biochem. Mol. Biol., 160, 169–174.
Drouin, J., Sun, Y. L., Chamberland, M., Gauthier, Y., De Lean, A., Nemer, M., and Schmidt, T. J. (1993) Novel glucocorticoid receptor complex with DNA element of the hormone-repressed POMC gene, EMBO J., 12, 145–156.
Malkoski, S. P., and Dorin, R. I. (1999) Composite glucocorticoid regulation at a functionally defined negative glucocorticoid response element of the human corticotropinreleasing hormone gene, Mol. Endocrinol., 13, 1629–1644.
Cohen, S., Janicki-Deverts, D., Doyle, W. J., Miller, G. E., Frank, E., Rabin, B. S., and Turner, R. B. (2012) Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk, Proc. Natl. Acad. Sci. USA, 109, 5995–5999.
Holsboer, F. (2000) The corticosteroid receptor hypothesis of depression, Neuropsychopharmacology, 23, 477–501.
Jarcho, M. R., Slavich, G. M., Tylova-Stein, H., Wolkowitz, O. M., and Burke, H. M. (2013) Dysregulated diurnal cortisol pattern is associated with glucocorticoid resistance in women with major depressive disorder, Biol. Psychol., 93, 150–158.
Pariante, C. M. (2009) Risk factors for development of depression and psychosis. Glucocorticoid receptors and pituitary implications for treatment with antidepressant and glucocorticoids, Ann. NY Acad. Sci., 1179, 144–152.
Pariante, C. M., and Miller, A. H. (2001) Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment, Biol. Psychiatry, 49, 391–404.
Naughton, M., Dinan, T. G., and Scott, L. V. (2014) Corticotropin-releasing hormone and the hypothalamicpituitary-adrenal axis in psychiatric disease, Handbook Clin. Neurol., 124, 69–91.
Watson, S., Gallagher, P., Del-Estal, D., Hearn, A., Ferrier, I. N., and Young, A. H. (2002) Hypothalamic-pituitary-adrenal axis function in patients with chronic depression, Psychol. Med., 32, 1021–1028.
Zobel, A. W., Nickel, T., Sonntag, A., Uhr, M., Holsboer, F., and Ising, M. (2001) Cortisol response in the combined dexamethasone/CRH test as predictor of relapse in patients with remitted depression. A prospective study, J. Psychiatr. Res., 35, 83–94.
Binder, E. B. (2009) The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders, Psychoneuroendocrinology, 34, Suppl. 1, S186–195.
Pariante, C. M., and Lightman, S. L. (2008) The HPA axis in major depression: classical theories and new developments, Trends Neurosci., 31, 464–468.
Hall, B. S., Moda, R. N., and Liston, C. (2015) Glucocorticoid mechanisms of functional connectivity changes in stress-related neuropsychiatric disorders, Neurobiol. Stress, 1, 174–183.
Jacobson, L., and Sapolsky, R. (1991) The role of the hippocampus in feedback regulation of the hypothalamicpituitary-adrenocortical axis, Endocr. Rev., 12, 118–134.
Sousa, N., Lukoyanov, N. V., Madeira, M. D., Almeida, O. F., and Paula-Barbosa, M. M. (2000) Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement, Neuroscience, 97, 253–266.
Vyas, A., Mitra, R., Shankaranarayana Rao, B. S., and Chattarji, S. (2002) Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons, J. Neurosci., 22, 6810–6818.
Vyas, S., Rodrigues, A. J., Silva, J. M., Tronche, F., Almeida, O. F., Sousa, N., and Sotiropoulos, I. (2016) Chronic stress and glucocorticoids: from neuronal plasticity to neurodegeneration, Neural Plast., 2016, 6391686.
Gould, E., McEwen, B. S., Tanapat, P., Galea, L. A., and Fuchs, E. (1997) Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation, J. Neurosci., 17, 2492–2498.
Shors, T. J. (2006) Significant life events and the shape of memories to come: a hypothesis, Neurobiol. Learn. Mem., 85, 103–115.
Anacker, C., Zunszain, P. A., Carvalho, L. A., and Pariante, C. M. (2011) The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology, 36, 415–425.
Pariante, C. M. (2006) The glucocorticoid receptor: part of the solution or part of the problem? J. Psychopharmacol., 20, 79–84.
Lopez, J. F., Chalmers, D. T., Little, K. Y., and Watson, S. J. (1998) A. E. Bennett Research Award. Regulation of serotonin 1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression, Biol. Psychiatry, 43, 547–573.
Alt, S. R., Turner, J. D., Klok, M. D., Meijer, O. C., Lakke, E. A., Derijk, R. H., and Muller, C. P. (2010) Differential expression of glucocorticoid receptor transcripts in major depressive disorder is not epigenetically programmed, Psychoneuroendocrinology, 35, 544–556.
Webster, M. J., Knable, M. B., O’Grady, J., Orthmann, J., and Weickert, C. S. (2002) Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders, Mol. Psychiatry, 7, 924, 985–994.
Perlman, W. R., Webster, M. J., Kleinman, J. E., and Weickert, C. S. (2004) Reduced glucocorticoid and estrogen receptor alpha messenger ribonucleic acid levels in the amygdala of patients with major mental illness, Biol. Psychiatry, 56, 844–852.
Chang, L. C., Jamain, S., Lin, C. W., Rujescu, D., Tseng, G. C., and Sibille, E. (2014) A conserved BDNF, glutamateand GABA-enriched gene module related to human depression identified by coexpression meta-analysis and DNA variant genome-wide association studies, PLoS One, 9, e90980.
Akula, N., Barb, J., Jiang, X., Wendland, J. R., Choi, K. H., Sen, S. K., Hou, L., Chen, D. T., Laje, G., Johnson, K., Lipska, B. K., Kleinman, J. E., Corrada-Bravo, H., Detera-Wadleigh, S., Munson, P. J., and McMahon, F. J. (2014) RNA-sequencing of the brain transcriptome implicates dysregulation of neuroplasticity, circadian rhythms and GTPase binding in bipolar disorder, Mol. Psychiatry, 19, 1179–1185.
Oakley, R. H., and Cidlowski, J. A. (2011) Cellular processing of the glucocorticoid receptor gene and protein: new mechanisms for generating tissue-specific actions of glucocorticoids, J. Biol. Chem., 286, 3177–3184.
Merkulov, V. M., and Merkulova, T. I. (2011) Isoforms of glucocorticoid receptor formed by alternative splicing and usage of alternative mRNA translation starts, Vavilov Zh. Genet. Selekt., 4, 621–632.
Oakley, R. H., Sar, M., and Cidlowski, J. A. (1996) The human glucocorticoid receptor beta isoform. Expression, biochemical properties, and putative function, J. Biol. Chem., 271, 9550–9559.
Kino, T., Manoli, I., Kelkar, S., Wang, Y., Su, Y. A., and Chrousos, G. P. (2009) Glucocorticoid receptor (GR) beta has intrinsic, GRalpha-independent transcriptional activity, Biochem. Biophys. Res. Commun., 381, 671–675.
Lewis-Tuffin, L. J., Jewell, C. M., Bienstock, R. J., Collins, J. B., and Cidlowski, J. A. (2007) Human glucocorticoid receptor beta binds RU-486 and is transcriptionally active, Mol. Cell Biol., 27, 2266–2282.
Pujols, L., Mullol, J., Roca-Ferrer, J., Torrego, A., Xaubet, A., Cidlowski, J. A., and Picado, C. (2002) Expression of glucocorticoid receptor alphaand beta-isoforms in human cells and tissues, Am. J. Physiol. Cell Physiol., 283, 1324–1331.
Webster, J. C., Oakley, R. H., Jewell, C. M., and Cidlowski, J. A. (2001) Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative beta isoform: a mechanism for the generation of glucocorticoid resistance, Proc. Natl. Acad. Sci. USA, 98, 6865–6870.
Lewis-Tuffin, L. J., and Cidlowski, J. A. (2006) The physiology of human glucocorticoid receptor beta (hGRbeta) and glucocorticoid resistance, Ann. NY Acad. Sci., 1069, 1–9.
Moalli, P. A., Pillay, S., Krett, N. L., and Rosen, S. T. (1993) Alternatively spliced glucocorticoid receptor messenger RNAs in glucocorticoid-resistant human multiple myeloma cells, Cancer Res., 53, 3877–3879.
Pandey, G. N., Rizavi, H. S., Ren, X., Dwivedi, Y., and Palkovits, M. (2013) Region-specific alterations in glucocorticoid receptor expression in the postmortem brain of teenage suicide victims, Psychoneuroendocrinology, 38, 2628–2639.
Szczepankiewicz, A., Leszczynska-Rodziewicz, A., Pawlak, J., Rajewska-Rager, A., Dmitrzak-Weglarz, M., Wilkosc, M., Skibinska, M., and Hauser, J. (2011) Glucocorticoid receptor polymorphism is associated with major depression and predominance of depression in the course of bipolar disorder, J. Affect. Disord., 134, 138–144.
Derijk, R. H., Schaaf, M. J., Turner, G., Datson, N. A., Vreugdenhil, E., Cidlowski, J., De Kloet, E. R., Emery, P., Sternberg, E. M., and Detera-Wadleigh, S. D. (2001) A human glucocorticoid receptor gene variant that increases the stability of the glucocorticoid receptor beta-isoform mRNA is associated with rheumatoid arthritis, J. Rheumatol., 28, 2383–2388.
Van Rossum, E. F., Binder, E. B., Majer, M., Koper, J. W., Ising, M., Modell, S., Salyakina, D., Lamberts, S. W., and Holsboer, F. (2006) Polymorphisms of the glucocorticoid receptor gene and major depression, Biol. Psychiatry, 59, 681–688.
Van Rossum, E. F., and Lamberts, S. W. (2004) Polymorphisms in the glucocorticoid receptor gene and their associations with metabolic parameters and body composition, Recent Prog. Horm. Res., 59, 333–357.
Russcher, H., Van Rossum, E. F., De Jong, F. H., Brinkmann, A. O., Lamberts, S. W., and Koper, J. W. (2005) Increased expression of the glucocorticoid receptorA translational isoform as a result of the ER22/23EK polymorphism, Mol. Endocrinol., 19, 1687–1696.
Kochetov, A. V., Merkulova, T. I., and Merkulov, V. M. (2012) Possible link between the synthesis of GR alpha isoforms and eIF2 alpha phosphorylation state, Med. Hypotheses, 79, 709–712.
Cao-Lei, L., Leija, S. C., Kumsta, R., Wust, S., Meyer, J., Turner, J. D., and Muller, C. P. (2011) Transcriptional control of the human glucocorticoid receptor: identification and analysis of alternative promoter regions, Hum. Genet., 129, 533–543.
Turner, J. D., Vernocchi, S., Schmitz, S., and Muller, C. P. (2014) Role of the 5'-untranslated regions in post-transcriptional regulation of the human glucocorticoid receptor, Biochim. Biophys. Acta, 1839, 1051–1061.
Weaver, I. C., Cervoni, N., Champagne, F. A., D’Alessio, A. C., Sharma, S., Seckl, J. R., Dymov, S., Szyf, M., and Meaney, M. J. (2004) Epigenetic programming by maternal behavior, Nat. Neurosci., 7, 847–854.
Mueller, B. R., and Bale, T. L. (2008) Sex-specific programming of offspring emotionality after stress early in pregnancy, J. Neurosci., 28, 9055–9065.
DeRijk, R. H., Schaaf, M., and De Kloet, E. R. (2002) Glucocorticoid receptor variants: clinical implications, J. Steroid Biochem. Mol. Biol., 81, 103–122.
Ismaili, N., and Garabedian, M. J. (2004) Modulation of glucocorticoid receptor function via phosphorylation, Ann. NY Acad. Sci., 1024, 86–101.
Ford, J., McEwan, I. J., Wright, A. P., and Gustafsson, J. A. (1997) Involvement of the transcription factor IID protein complex in gene activation by the N-terminal transactivation domain of the glucocorticoid receptor in vitro, Mol. Endocrinol., 11, 1467–1475.
Robyr, D., Wolffe, A. P., and Wahli, W. (2000) Nuclear hormone receptor coregulators in action: diversity for shared tasks, Mol. Endocrinol., 14, 329–347.
Chen, W., Dang, T., Blind, R. D., Wang, Z., Cavasotto, C. N., Hittelman, A. B., Rogatsky, I., Logan, S. K., and Garabedian, M. J. (2008) Glucocorticoid receptor phosphorylation differentially affects target gene expression, Mol. Endocrinol., 22, 1754–1766.
Anacker, C., Cattaneo, A., Musaelyan, K., Zunszain, P. A., Horowitz, M., Molteni, R., Luoni, A., Calabrese, F., Tansey, K., Gennarelli, M., Thuret, S., Price, J., Uher, R., Riva, M. A., and Pariante, C. M. (2013) Role for the kinase SGK1 in stress, depression, and glucocorticoid effects on hippocampal neurogenesis, Proc. Natl. Acad. Sci. USA, 110, 8708–8713.
Itoh, M., Adachi, M., Yasui, H., Takekawa, M., Tanaka, H., and Imai, K. (2002) Nuclear export of glucocorticoid receptor is enhanced by c-Jun N-terminal kinase-mediated phosphorylation, Mol. Endocrinol., 16, 2382–2392.
Miller, A. L., Webb, M. S., Copik, A. J., Wang, Y., Johnson, B. H., Kumar, R., and Thompson, E. B. (2005) p38 mitogen-activated protein kinase (MAPK) is a key mediator in glucocorticoid-induced apoptosis of lymphoid cells: correlation between p38 MAPK activation and site-specific phosphorylation of the human glucocorticoid receptor at serine 211, Mol. Endocrinol., 19, 1569–1583.
Jovicic, M. J., Lukic, I., Radojcic, M., Adzic, M., and Maric, N. P. (2015) Modulation of c-Jun N-terminal kinase signaling and specific glucocorticoid receptor phosphorylation in the treatment of major depression, Med. Hypotheses, 85, 291–294.
Mitic, M., Simic, I., Djordjevic, J., Radojcic, M. B., and Adzic, M. (2013) Gender-specific effects of fluoxetine on hippocampal glucocorticoid receptor phosphorylation and behavior in chronically stressed rats, Neuropharmacology, 70, 100–111.
Lee, M. S., Park, W. S., Kim, Y. H., Kwon, S. H., Jang, Y. J., Han, D., Morita, K., and Her, S. (2013) Antidepressantlike effects of cortex mori radicis extract via bidirectional phosphorylation of glucocorticoid receptors in the hippocampus, Behav. Brain Res., 236, 56–61.
Simic, I., Maric, N. P., Mitic, M., Soldatovic, I., Pavlovic, Z., Mihaljevic, M., Andric, S., Radojcic, M. B., and Adzic, M. (2013) Phosphorylation of leukocyte glucocorticoid receptor in patients with current episode of major depressive disorder, Prog. Neuropsychopharmacol. Biol. Psychiatry, 40, 281–285.
Echeverria, P. C., and Picard, D. (2010) Molecular chaperones, essential partners of steroid hormone receptors for activity and mobility, Biochim. Biophys. Acta, 1803, 641–649.
Cheung, J., and Smith, D. F. (2000) Molecular chaperone interactions with steroid receptors: an update, Mol. Endocrinol., 14, 939–946.
Pratt, W. B., Galigniana, M. D., Morishima, Y., and Murphy, P. J. (2004) Role of molecular chaperones in steroid receptor action, Essays Biochem., 40, 41–58.
Davies, T. H., Ning, Y. M., and Sanchez, E. R. (2002) A new first step in activation of steroid receptors: hormoneinduced switching of FKBP51 and FKBP52 immunophilins, J. Biol. Chem., 277, 4597–4600.
Harrell, J. M., Murphy, P. J., Morishima, Y., Chen, H., Mansfield, J. F., Galigniana, M. D., and Pratt, W. B. (2004) Evidence for glucocorticoid receptor transport on microtubules by dynein, J. Biol. Chem., 279, 54647–54654.
Billing, A. M., Fack, F., Renaut, J., Olinger, C. M., Schote, A. B., Turner, J. D., and Muller, C. P. (2007) Proteomic analysis of the cortisol-mediated stress response in THP-1 monocytes using DIGE technology, J. Mass Spectrom., 42, 1433–1444.
U, M., Shen, L., Oshida, T., Miyauchi, J., Yamada, M., and Miyashita, T. (2004) Identification of novel direct transcriptional targets of glucocorticoid receptor, Leukemia, 18, 1850–1856.
Vermeer, H., Hendriks-Stegeman, B. I., Van der Burg, B., Van Buul-Offers, S. C., and Jansen, M. (2003) Glucocorticoid-induced increase in lymphocytic FKBP51 messenger ribonucleic acid expression: a potential marker for glucocorticoid sensitivity, potency, and bioavailability, J. Clin. Endocrinol. Metab., 88, 277–284.
Woodruff, P. G., Boushey, H. A., Dolganov, G. M., Barker, C. S., Yang, Y. H., Donnelly, S., Ellwanger, A., Sidhu, S. S., Dao-Pick, T. P., Pantoja, C., Erle, D. J., Yamamoto, K. R., and Fahy, J. V. (2007) Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids, Proc. Natl. Acad. Sci. USA, 104, 15858–15863.
Lee, R. S., Tamashiro, K. L., Yang, X., Purcell, R. H., Harvey, A., Willour, V. L., Huo, Y., Rongione, M., Wand, G. S., and Potash, J. B. (2010) Chronic corticosterone exposure increases expression and decreases deoxyribonucleic acid methylation of Fkbp5 in mice, Endocrinology, 151, 4332–4343.
Scharf, S. H., Liebl, C., Binder, E. B., Schmidt, M. V., and Muller, M. B. (2011) Expression and regulation of the Fkbp5 gene in the adult mouse brain, PLoS One, 6, e16883.
Hubler, T. R., and Scammell, J. G. (2004) Intronic hormone response elements mediate regulation of FKBP5 by progestins and glucocorticoids, Cell Stress Chaperones, 9, 243–252.
Reddy, T. E., Pauli, F., Sprouse, R. O., Neff, N. F., Newberry, K. M., Garabedian, M. J., and Myers, R. M. (2009) Genomic determination of the glucocorticoid response reveals unexpected mechanisms of gene regulation, Genome Res., 19, 2163–2171.
Denny, W. B., Prapapanich, V., Smith, D. F., and Scammell, J. G. (2005) Structure-function analysis of squirrel monkey FK506-binding protein 51, a potent inhibitor of glucocorticoid receptor activity, Endocrinology, 146, 3194–3201.
Klengel, T., Mehta, D., Anacker, C., Rex-Haffner, M., Pruessner, J. C., Pariante, C. M., Pace, T. W., Mercer, K. B., Mayberg, H. S., Bradley, B., Nemeroff, C. B., Holsboer, F., Heim, C. M., Ressler, K. J., Rein, T., and Binder, E. B. (2013) Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions, Nat. Neurosci., 16, 33–41.
Chrousos, G. P., Renquist, D., Brandon, D., Eil, C., Pugeat, M., Vigersky, R., Cutler, G. B., Jr., Loriaux, D. L., and Lipsett, M. B. (1982) Glucocorticoid hormone resistance during primate evolution: receptor-mediated mechanisms, Proc. Natl. Acad. Sci. USA, 79, 2036–2040.
Reynolds, P. D., Ruan, Y., Smith, D. F., and Scammell, J. G. (1999) Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51, J. Clin. Endocrinol. Metab., 84, 663–669.
Scammell, J. G., Denny, W. B., Valentine, D. L., and Smith, D. F. (2001) Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates, Gen. Comp. Endocrinol., 124, 152–165.
Denny, W. B., Valentine, D. L., Reynolds, P. D., Smith, D. F., and Scammell, J. G. (2000) Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding, Endocrinology, 141, 4107–4113.
Guidotti, G., Calabrese, F., Anacker, C., Racagni, G., Pariante, C. M., and Riva, M. A. (2013) Glucocorticoid receptor and FKBP5 expression is altered following exposure to chronic stress: modulation by antidepressant treatment, Neuropsychopharmacology, 38, 616–627.
Xing, Y., Hou, J., Meng, Q., Yang, M., Kurihara, H., and Tian, J. (2015) Novel antidepressant candidate RO-05 modulated glucocorticoid receptors activation and FKBP5 expression in chronic mild stress model in rats, Neuroscience, 290, 255–265.
Pei, H., Li, L., Fridley, B. L., Jenkins, G. D., Kalari, K. R., Lingle, W., Petersen, G., Lou, Z., and Wang, L. (2009) FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt, Cancer Cell, 16, 259–266.
Beaulieu, J. M., Gainetdinov, R. R., and Caron, M. G. (2009) Akt/GSK3 signaling in the action of psychotropic drugs, Annu. Rev. Pharmacol. Toxicol., 49, 327–347.
Charney, D. S., and Manji, H. K. (2004) Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention, Science’s STKE, 2004, re5.
Duman, R. S., and Voleti, B. (2012) Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents, Trends Neurosci., 35, 47–56.
Binder, E. B., Salyakina, D., Lichtner, P., Wochnik, G. M., Ising, M., Putz, B., Papiol, S., Seaman, S., Lucae, S., Kohli, M. A., Nickel, T., Kunzel, H. E., Fuchs, B., Majer, M., Pfennig, A., Kern, N., Brunner, J., Modell, S., Baghai, T., Deiml, T., Zill, P., Bondy, B., Rupprecht, R., Messer, T., Kohnlein, O., Dabitz, H., Bruckl, T., Muller, N., Pfister, H., Lieb, R., Mueller, J. C., Lohmussaar, E., Strom, T. M., Bettecken, T., Meitinger, T., Uhr, M., Rein, T., Holsboer, F., and Muller-Myhsok, B. (2004) Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment, Nat. Genet., 36, 1319–1325.
Brent, D., Melhem, N., Ferrell, R., Emslie, G., Wagner, K. D., Ryan, N., Vitiello, B., Birmaher, B., Mayes, T., Zelazny, J., Onorato, M., Devlin, B., Clarke, G., DeBar, L., and Keller, M. (2010) Association of FKBP5 polymorphisms with suicidal events in the Treatment of Resistant Depression in Adolescents (TORDIA) study, Am. J. Psychiatry, 167, 190–197.
Lavebratt, C., Aberg, E., Sjoholm, L. K., and Forsell, Y. (2010) Variations in FKBP5 and BDNF genes are suggestively associated with depression in a Swedish populationbased cohort, J. Affect. Disord., 125, 249–255.
Lekman, M., Laje, G., Charney, D., Rush, A. J., Wilson, A. F., Sorant, A. J., Lipsky, R., Wisniewski, S. R., Manji, H., McMahon, F. J., and Paddock, S. (2008) The FKBP5 gene in depression and treatment response–an association study in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) cohort, Biol. Psychiatry, 63, 1103–1110.
Papiol, S., Arias, B., Gasto, C., Gutierrez, B., Catalan, R., and Fananas, L. (2007) Genetic variability at HPA axis in major depression and clinical response to antidepressant treatment, J. Affect. Disord., 104, 83–90.
Ising, M., Depping, A. M., Siebertz, A., Lucae, S., Unschuld, P. G., Kloiber, S., Horstmann, S., Uhr, M., Muller-Myhsok, B., and Holsboer, F. (2008) Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls, Eur. J. Neurosci., 28, 389–398.
Stamm, T. J., Rampp, C., Wiethoff, K., Stingl, J., Mossner, R., O’Malley, G., Ricken, R., Seemuller, F., Keck, M., Fisher, R., Gaebel, W., Maier, W., Moller, H. J., Bauer, M., and Adli, M. (2016) The FKBP5 polymorphism rs1360780 influences the effect of an algorithmbased antidepressant treatment and is associated with remission in patients with major depression, J. Psychopharmacol., 30, 40–47.
Menke, A., Klengel, T., Rubel, J., Bruckl, T., Pfister, H., Lucae, S., Uhr, M., Holsboer, F., and Binder, E. B. (2013) Genetic variation in FKBP5 associated with the extent of stress hormone dysregulation in major depression, Genes Brain Behav., 12, 289–296.
Hohne, N., Poidinger, M., Merz, F., Pfister, H., Bruckl, T., Zimmermann, P., Uhr, M., Holsboer, F., and Ising, M. (2015) FKBP5 genotype-dependent DNA methylation and mRNA regulation after psychosocial stress in remitted depression and healthy controls, Int. J. Neuropsychopharmacol., 18.
Abramovitz, L., Shapira, T., Ben-Dror, I., Dror, V., Granot, L., Rousso, T., Landoy, E., Blau, L., Thiel, G., and Vardimon, L. (2008) Dual role of NRSF/REST in activation and repression of the glucocorticoid response, J. Biol. Chem., 283, 110–119.
Merkulova, T. I., Ananko, E. A., Ignat’eva, E. V., and Kolchanov, N. A. (2013) Regulatory transcription codes in eukaryotic genomes, Genetika, 49, 37–54.
Gaali, S., Kirschner, A., Cuboni, S., Hartmann, J., Kozany, C., Balsevich, G., Namendorf, C., FernandezVizarra, P., Sippel, C., Zannas, A. S., Draenert, R., Binder, E. B., Almeida, O. F., Ruhter, G., Uhr, M., Schmidt, M. V., Touma, C., Bracher, A., and Hausch, F. (2015) Selective inhibitors of the FK506-binding protein 51 by induced fit, Nat. Chem. Biol., 11, 33–37.
Nicolaides, N. C., Galata, Z., Kino, T., Chrousos, G. P., and Charmandari, E. (2010) The human glucocorticoid receptor: molecular basis of biologic function, Steroids, 75, 1–12.
King, H. A., Trotter, K. W., and Archer, T. K. (2012) Chromatin remodeling during glucocorticoid receptor regulated transactivation, Biochim. Biophys. Acta, 1819, 716–726.
Wu, J. N., Pinello, L., Yissachar, E., Wischhusen, J. W., Yuan, G. C., and Roberts, C. W. (2015) Functionally distinct patterns of nucleosome remodeling at enhancers in glucocorticoid-treated acute lymphoblastic leukemia, Epigenetics Chromatin, 8, 53.
Swinstead, E. E., Paakinaho, V., Presman, D. M., and Hager, G. L. (2016) Pioneer factors and ATP-dependent chromatin remodeling factors interact dynamically: a new perspective–multiple transcription factors can effect chromatin pioneer functions through dynamic interactions with ATP-dependent chromatin remodeling factors, Bioessays, 38, 1150–1157.
Hervouet, E., Vallette, F. M., and Cartron, P. F. (2010) Dnmt1/transcription factor interactions: an alternative mechanism of DNA methylation inheritance, Genes Cancer, 1, 434–443.
Burkhart, B. A., Ivey, M. L., and Archer, T. K. (2009) Long-term low level glucocorticoid exposure induces persistent repression in chromatin, Mol. Cell Endocrinol., 298, 66–75.
Nestler, E. J., Pena, C. J., Kundakovic, M., Mitchell, A., and Akbarian, S. (2016) Epigenetic basis of mental illness, Neuroscientist, 22, 447–463.
Pena, C. J., Bagot, R. C., Labonte, B., and Nestler, E. J. (2014) Epigenetic signaling in psychiatric disorders, J. Mol. Biol., 426, 3389–3412.
Kundakovic, M., and Champagne, F. A. (2015) Early-life experience, epigenetics, and the developing brain, Neuropsychopharmacology, 40, 141–153.
McGowan, P. O., Suderman, M., Sasaki, A., Huang, T. C., Hallett, M., Meaney, M. J., and Szyf, M. (2011) Broad epigenetic signature of maternal care in the brain of adult rats, PLoS One, 6, e14739.
Lo, P. K., and Sukumar, S. (2008) Epigenomics and breast cancer, Pharmacogenomics, 9, 1879–1902.
LaPlant, Q., Vialou, V., Covington, H. E., 3rd, Dumitriu, D., Feng, J., Warren, B. L., Maze, I., Dietz, D. M., Watts, E. L., Iniguez, S. D., Koo, J. W., Mouzon, E., Renthal, W., Hollis, F., Wang, H., Noonan, M. A., Ren, Y., Eisch, A. J., Bolanos, C. A., Kabbaj, M., Xiao, G., Neve, R. L., Hurd, Y. L., Oosting, R. S., Fan, G., Morrison, J. H., and Nestler, E. J. (2010) Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens, Nat. Neurosci., 13, 1137–1143.
Sales, A. J., Biojone, C., Terceti, M. S., Guimaraes, F. S., Gomes, M. V., and Joca, S. R. (2011) Antidepressant-like effect induced by systemic and intra-hippocampal administration of DNA methylation inhibitors, Br. J. Pharmacol., 164, 1711–1721.
Xing, B., Liu, P., Xu, W. J., Xu, F. Y., and Dang, Y. H. (2014) Effect of microinjecting of 5-aza-2-deoxycytidine into ventrolateral orbital cortex on depressive-like behavior in rats, Neurosci. Lett., 574, 11–14.
Poulter, M. O., Du, L., Weaver, I. C., Palkovits, M., Faludi, G., Merali, Z., Szyf, M., and Anisman, H. (2008) GABAA receptor promoter hypermethylation in suicide brain: implications for the involvement of epigenetic processes, Biol. Psychiatry, 64, 645–652.
Higuchi, F., Uchida, S., Yamagata, H., Otsuki, K., Hobara, T., Abe, N., Shibata, T., and Watanabe, Y. (2011) State-dependent changes in the expression of DNA methyltransferases in mood disorder patients, J. Psychiatr. Res., 45, 1295–1300.
Bose, R., Spulber, S., Kilian, P., Heldring, N., Lonnerberg, P., Johnsson, A., Conti, M., Hermanson, O., and Ceccatelli, S. (2015) Tet3 mediates stable glucocorticoid-induced alterations in DNA methylation and Dnmt3a/Dkk1 expression in neural progenitors, Cell Death Dis., 6, e1793.
Bose, R., Moors, M., Tofighi, R., Cascante, A., Hermanson, O., and Ceccatelli, S. (2010) Glucocorticoids induce long-lasting effects in neural stem cells resulting in senescence-related alterations, Cell Death Dis., 1, e92.
Covington, H. E., 3rd, Maze, I., Vialou, V., and Nestler, E. J. (2015) Antidepressant action of HDAC inhibition in the prefrontal cortex, Neuroscience, 298, 329–335.
Jenuwein, T., and Allis, C. D. (2001) Translating the histone code, Science, 293, 1074–1080.
Hunter, R. G., McCarthy, K. J., Milne, T. A., Pfaff, D. W., and McEwen, B. S. (2009) Regulation of hippocampal H3 histone methylation by acute and chronic stress, Proc. Natl. Acad. Sci. USA, 106, 20912–20917.
Hunter, R. G., Murakami, G., Dewell, S., Seligsohn, M., Baker, M. E., Datson, N. A., McEwen, B. S., and Pfaff, D. W. (2012) Acute stress and hippocampal histone H3 lysine 9 trimethylation, a retrotransposon silencing response, Proc. Natl. Acad. Sci. USA, 109, 17657–17662.
Kenworthy, C. A., Sengupta, A., Luz, S. M., Ver Hoeve, E. S., Meda, K., Bhatnagar, S., and Abel, T. (2014) Social defeat induces changes in histone acetylation and expression of histone modifying enzymes in the ventral hippocampus, prefrontal cortex, and dorsal raphe nucleus, Neuroscience, 264, 88–98.
Covington, H. E., 3rd, Maze, I., Sun, H., Bomze, H. M., DeMaio, K. D., Wu, E. Y., Dietz, D. M., Lobo, M. K., Ghose, S., Mouzon, E., Neve, R. L., Tamminga, C. A., and Nestler, E. J. (2011) A role for repressive histone methylation in cocaine-induced vulnerability to stress, Neuron, 71, 656–670.
Robison, A. J., Vialou, V., Sun, H. S., Labonte, B., Golden, S. A., Dias, C., Turecki, G., Tamminga, C., Russo, S., Mazei-Robison, M., and Nestler, E. J. (2014) Fluoxetine epigenetically alters the CaMKIIalpha promoter in nucleus accumbens to regulate DeltaFosB binding and antidepressant effects, Neuropsychopharmacology, 39, 1178–1186.
Covington, H. E., 3rd, Maze, I., LaPlant, Q. C., Vialou, V. F., Ohnishi, Y. N., Berton, O., Fass, D. M., Renthal, W., Rush, A. J., 3rd, Wu, E. Y., Ghose, S., Krishnan, V., Russo, S. J., Tamminga, C., Haggarty, S. J., and Nestler, E. J. (2009) Antidepressant actions of histone deacetylase inhibitors, J. Neurosci., 29, 11451–11460.
Tsankova, N. M., Berton, O., Renthal, W., Kumar, A., Neve, R. L., and Nestler, E. J. (2006) Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action, Nat. Neurosci., 9, 519–525.
Hierholzer, K., and Buhler, H. (1996) Metabolism of cortical steroid hormones and their general mode of action, in Comprehensive Human Physiology (Greger, R., and Windhorst, U., eds.) Springer-Verlag, Heidelberg, pp. 79–93.
Sapolsky, R. M., Romero, L. M., and Munck, A. U. (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions, Endocrin. Rev., 21, 55–89.
Charmandari, E., Tsigos, C., and Chrousos, G. (2005) Endocrinology of the stress response, Annu. Rev. Physiol., 67, 259–284.
Smith, S. M., and Vale, W. W. (2006) The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress, Dialogues Clin. Neurosci., 8, 383–395.
Holsboer, F., and Barden, N. (1996) Antidepressants and hypothalamic-pituitary-adrenocortical regulation, Endocrin. Rev., 17, 187–205.
Arango-Lievano, M., and Jeanneteau, F. (2016) Timing and crosstalk of glucocorticoid signaling with cytokines, neurotransmitters and growth factors, Pharmacol. Res., 113, 1–17.
Kim, Y. K., Na, K. S., Myint, A. M., and Leonard, B. E. (2016) The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression, Prog. Neuropsychopharmacol. Biol. Psychiatry, 64, 277–284.
Grigoryan, G. A., Dygalo, N. N., Gekht, A. B., Stepanichev, M. Yu., and Gulyaeva, N. V. (2014) Molecular and cellular mechanisms of depression: role of glucocorticoids, cytokines, nuerotransmitters, and trophic factors in genesis of depressive disorders, Usp. Fiziol. Nauk, 45, 3–19.
Jeon, S. W., and Kim, Y. K. (2016) Neuroinflammation and cytokine abnormality in major depression: cause or consequence in that illness? World J. Psychiatry, 6, 283–293.
Reus, G. Z., Fries, G. R., Stertz, L., Badawy, M., Passos, I. C., Barichello, T., Kapczinski, F., and Quevedo, J. (2015) The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders, Neuroscience, 300, 141–154.
Hong, H., Kim, B. S., and Im, H. I. (2016) Pathophysiological role of neuroinflammation in neurodegenerative diseases and psychiatric disorders, Int. Neurourol. J., 20, S2–7.
De Kloet, E. R., De Jong, I. E., and Oitzl, M. S. (2008) Neuropharmacology of glucocorticoids: focus on emotion, cognition and cocaine, Eur. J. Pharmacol., 585, 473–482.
Fietta, P., and Delsante, G. (2009) Central nervous system effects of natural and synthetic glucocorticoids, Psychiatry Clin. Neurosci., 63, 613–622.
Finsterwald, C., and Alberini, C. M. (2014) Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: from adaptive responses to psychopathologies, Neurobiol. Learn. Mem., 112, 17–29.
Kino, T. (2015) Stress, glucocorticoid hormones, and hippocampal neural progenitor cells: implications to mood disorders, Front. Physiol., 6, 230.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V. M. Merkulov, T. I. Merkulova, N. P. Bondar, 2017, published in Biokhimiya, 2017, Vol. 82, No. 3, pp. 494-510.
Rights and permissions
About this article
Cite this article
Merkulov, V.M., Merkulova, T.I. & Bondar, N.P. Mechanisms of brain glucocorticoid resistance in stress-induced psychopathologies. Biochemistry Moscow 82, 351–365 (2017). https://doi.org/10.1134/S0006297917030142
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297917030142