Abstract
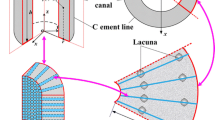
The pathway for intracortical fluid flow response to a step-load was identified in vivo using intramedullary pressure (ImP) and streaming potential (SP) measurements, and allowed the development of a load-induced flow mechanism which considers mechanotransduction and mechanoelectrotransduction phenomena. An avian model was used for monitoring, simultaneously, ImP and SP under axial loading which generated peak strains of approximately 600 microstrain (με). ImP response to step-load decayed more quickly than SP relaxation, in which multiple time constants were observed during the relaxations. While the initial relaxation of SP showed a decay on the order of 200 ms, ImP decayed on the order of approximately 100 ms. After the initial decay (∼200 ms after loading), ImP quickly relaxed to base line, while SP continued to dominate relaxation. It appears that the decay of ImP is indicative of resistive fluid flow occurring primarily in the vasculature and other intraosseous channels such as lacunar-canalicular pores, and that SP represents the fluid flow in the smaller porosities, i.e., lacunar-canalicular system or even microspores. These results suggest that SP and ImP decays are determined by a hierarchical interdependent system of multiple porosities, and that the temporal dynamics of load-bearing define the manner in which the fluid patterns and pressures are distributed. © 2002 Biomedical Engineering Society.
PAC2002: 8719Tt, 8719Rr, 8719Nn
Similar content being viewed by others
REFERENCES
Chole, R. A., and S. P. Tinling. Incomplete coverage of mammalian bone cell matrix by lining cells. Ann. Otol. Rhinol. Laryngol. 102:543–550, 1993.
Cochran, G. V., M. W. Johnson, M. P. Kadaba, F. Vosburgh, M. W. Ferguson-Pell, and V. R. Palmieri. Piezoelectric internal fixation devices: A new approach to electrical augmentation of osteogenesis. J. Orthop. Res. 3:508–513, 1985.
Cowin, S. C. Bone poroelasticity. J. Biomech. 32:217–238, 1999.
Cowin, S. C., S. Weinbaum, and Y. Zeng. A case for bone canaliculi as the anatomical site of strain generated potentials. J. Biomech. 28:1281–1297, 1995.
Dillaman, R. M. Movement of ferritin in the 2-day-old chick femur. Anat. Rec. 209:445–453, 1984.
Dillaman, R. M., R. D. Roer, and D. M. Gay. Fluid movement in bone: Theoretical and empirical. J. Biomech. 24:163–177, 1991.
Doty, S. D., and B. H. Schofield. Metabolic and structural changes with osteocytes of rat bone. In Calcium Parathyroid Hormone and the Calcitonins, edited by Talmage and Munson. Amsterdam: Excerpta Medica, 1972, pp. 353–364.
Frangos, J. A., T. Y. Huang, and C. B. Clark. Steady shear and step changes in shear stimulate endothelium via independent mechanisms-superposition of transient and sustained nitric oxide production. Biochem. Biophys. Res. Commun. 224:660–665, 1996.
Gross, D., and W. S. Williams. Streaming potential and the electromechanical response of physiologically moist bone. J. Biomech. 15:277–295, 1982.
Hillsley, M. V., and J. A. Frangos. Review: bone tissue engineering: The role of interstitial fluid flow. Biotechnol. Bioeng. 43:573–581, 1994.
Iannacone, W., E. Korostoff, and S. R. Pollack. Microelectrode study of stress-generated potentials obtained from uniform and nonuniform compression of human bone. J. Biomed. Mater. Res. 13:753–763, 1979.
Jacobs, C. R., C. E. Yellowley, B. R. Davis, Z. Zhou, J. M. Cimbala, and H. J. Donahue. Differential effect of steady versus oscillating flow on bone cells. J. Biomech. 31:969–976, 1998.
Johnson, M. W. Behavior of fluid in stressed bone and cellular stimulation. Calcif. Tissue Int. 36:S72-S76, 1984.
Johnson, M. W., D. A. Chakkalakal, R. A. Harper, J. L. Katz, and S. W. Rouhana. Fluid flow in bone in vitro. J. Biomech. 15:881–885, 1982.
Kelly, P. J., K. N. An, E. Y. S. Chao, and J. A. Rand. Fracture healing: Biomechanical, fluid dynamic and electrical considerations. Bone Mineral Research. New York: Elsevier, 1985, pp. 295–319.
Li, G. P., J. T. Bronk, K. N. An, and P. J. Kelly. Permeability of cortical bone of canine tibiae. Microvasc. Res. 34:302–310, 1987.
Mak, A. F., D. T. Huang, J. D. Zhang, and P. Tong. Deformation-induced hierarchical flows and drag forces in bone canaliculi and matrix microporosity. J. Biomech. 30:11–18, 1997.
Mak, A. F., L. Qin, L. K. Hung, C. W. Cheng, and C. F. Tin. A histomorphometric observation of flows in cortical bone under dynamic loading. Microvasc. Res. 59:290–300, 2000.
Montgomery, R. J., B. D. Sutker, J. T. Bronk, S. R. Smith, and P. J. Kelly. Interstitial fluid flow in cortical bone. Microvasc. Res. 35:295–307, 1988.
Morris, M. A., J. A. Lopez-Curto, S. P. Hughes, K. N. An, J. B. Bassingthwaighte, and P. J. Kelly. Fluid spaces in canine bone and marrow. Microvasc. Res. 23:188–200, 1982.
Neuman, M. W. Blood:Bone equilibrium. Calcif. Tissue Int. 34:117–120, 1982.
Otter, M. W., and G. V. Cochran. Comments on "fluid movement in bone: Theoretical and empirical." J. Biomech. 25:1495, 1992.
Otter, M. W., V. R. Palmieri, and G. V. Cochran. Transcortical streaming potentials are generated by circulatory pressure gradients in living canine tibia. J. Orthop. Res. 8:119–126, 1990.
Otter, M. W., V. R. Palmieri, D. D. Wu, K. G. Seiz, L. A. MacGinitie, and G. V. Cochran. A comparative analysis of streaming potentials in vivo and in vitro. J. Orthop. Res. 10:710–719, 1992.
Otter, M. W., Y. X. Qin, C. T. Rubin, and K. J. McLeod. Does bone perfusion/reperfusion initiate bone remodeling and the stress fracture syndrome? Med. Hypotheses 53:363–368, 1999.
Otter, M. W., D. D. Wu, W. A. Bieber, and G. V. Cochran. Intraarterial protamine sulfate reduces the magnitude of streaming potentials in living canine tibia. Calcif. Tissue Int. 53:411–415, 1993.
Piekarski, K., and M. Munro. Transport mechanism operating between blood supply and osteocytes in long bones. Nature (London) 269:80–82, 1977.
Pienkowski, D., and S. R. Pollack. The origin of stressgenerated potentials in fluid-saturated bone. J. Orthop. Res. 1:30–41, 1983.
Pollack, S. R., N. Petrov, R. Salzstein, G. Brankov, and R. Blagoeva. An anatomical model for streaming potentials in osteons. J. Biomech. 17:627–636, 1984.
Pollack, S. R., R. Salzstein, and D. Pienkowski. Streaming potential in fluid filled bone. Ferroelectrics 60:297–309, 1984.
Qin, Y. X., K. McLeod, M. W. Otter, and C. T. Rubin. The interdependent role of loading frequency, intracortical fluid pressure and pressure gradients in guiding sitespecific bone adaptation. 44th Ann. Mtg. Orthop. Res. Soc. 23:544, 1998.
Qin, Y. X., K. McLeod, and C. T. Rubin, Intracortical fluid flow is induced by dynamic intramedullary pressure independent of matrix deformation. 46th Ann. Mtg. Orth. Res. Soc. 25:740, 2000.
Qin, Y. X., C. T. Rubin, and K. J. McLeod, Nonlinear dependence of loading intensity and cycle number in the maintenance of bone mass and morphology. J. Orthop. Res. 16:482–489, 1998.
Rubin, C. T., and L. E. Lanyon. Osteoregulatory nature of mechnaical stimuli: function as a determinant for adaptive remodeling in bone. J. Orthop. Res. 5:300–310, 1987.
Salzstein, R. A., and S. R. Pollack. Electromechanicall potentials in cortical bone—II. Experimental analysis. J. Biomech. 20:271–280, 1987.
Salzstein, R. A., S. R. Pollack, A. F. Mak, and N. Petrov, Electromechanical potentials in cortical bone-I. A continuum approach. J. Biomech 20:261–270, 1987.
Scher, H., M. F. Shlesinger, and J. T. Bendler, Time-scale invariance in transport and relaxation. Phys. Today 44:26–34, 1991.
Scott, G. C., and E. Korostoff, Oscillatory and step response electromechanical phenomena in human and bovine bone. J. Biomech. 23:127–143, 1990.
Seliger, W. G. Tissue fluid movement in compact bone. Anat. Rec. 166:247–255, 1970.
Smit, T. H., J. M. Huyghe, and S. C. Cowin. Estimation of the poroeleastic parameters of cortical bone. J. Biomech. (in press).
Soares, A. M., V. E. Arana-Chavez, A. R. Reid, and E. Katchburian, Lanthanum tracer and freeze-fracture studies suggest that compartmentalisation of early bone matrix may be related to initial mineralisation. J. Anat. 181 (Pt 2):345–356, 1992.
Starkebaum, W., S. R. Pollack, and E. Korostoff. Microelectrode studies of stress-generated potentials in four-point bending of bone. J. Biomed. Mater. Res. 13:729–751, 1979.
Tanaka, T., and A. Sakano. Differences in permeability of microperoxidase and horseradish peroxidase into the alveolar bone of developing rats. J. Dent. Res. 64:870–876, 1985.
Tate, M. L., and U. Knothe, An ex vivo model to study transport processes and fluid flow in loaded bone. J. Biomech. 33:247–254, 2000.
Tate, M. L., P. Niederer, and U. Knothe. In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone 2:107–117, 1998.
Wang, L., S. P. Fritton, S. C. Cowin, and S. Weinbaum. Fluid pressure relaxation depends upon osteonal microstructure: Modeling an oscillatory bending experiment. J. Biomech. 32:663–672, 1999.
Weinbaum, S., S. C. Cowin, and Y. Zeng. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J. Biomech. 27:339–360, 1994.
Weinbaum, S., P. Guo, and L. You. A new view of mechanotransduction and strain amplification in cells with microvilli and cell processes. Biorheology 38:119–142, 2001.
Zeng, Y., S. C. Cowin, and S. Weinbaum. A fiber matrix model for fluid flow and streaming potentials in the canaliculi of an osteon. Ann. Biomed. Eng. 22:280–292, 1994.
Zhang, D., and S. C. Cowin. Oscillatory bending of a poroelastic beam. J. Mech. Phys. Solids 42:1575–1579, 1994.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Qin, YX., Lin, W. & Rubin, C. The Pathway of Bone Fluid Flow as Defined by In Vivo Intramedullary Pressure and Streaming Potential Measurements. Annals of Biomedical Engineering 30, 693–702 (2002). https://doi.org/10.1114/1.1483863
Issue Date:
DOI: https://doi.org/10.1114/1.1483863