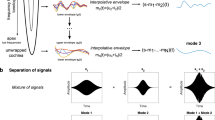
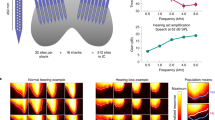
Abstract
We show that we can accurately model the auditory-nerve discharge patterns in response to sounds as complex as speech and ask how we may exploit this knowledge to test new strategies for hearing-aid signal processing. We describe the auditory-nerve representations of vowels in normal and noise-damaged ears. The normal representations are predicted well by a cochlear signal processing model originally developed by Carney (Carney, L. H. J. Acoust. Soc. Am. 93:401–417, 1993). Basilar-membrane tuning is represented by a time-varying narrow-band filter. Outer hair cell control of tuning is exerted by a nonlinear feedback path. We show that the effects of noise-induced outer hair cell damage can be modeled by scaling the feedback signal appropriately and use the model to test one strategy for hearing-aid speech processing. We conclude by discussing some aspects of future trends in biomedical engineering approaches to problems of hearing impairment. © 2002 Biomedical Engineering Society.
PAC2002: 4350-x, 4364Dw, 8780Xa, 4360Bf, 4366Ts, 8719La, 8710+e, 8717Aa, 8716Xa
Similar content being viewed by others
REFERENCES
Blackburn, C. C., and M. B. Sachs. The representations of the steady–state vowel sound / ?/ in the discharge patterns of cat anteroventral cochlear nucleus neurons. J. Neurophysiol. 63:1191–1212, 1990.
Brownell, W. E., C. R. Bader, D. Bertrand, and Y. de Ribaupierre. Evoked mechanical responses of isolated cochlear outer hair cells. Science 227(4683):194–196, 1985.
Carney, L. H. A model for the responses of low–frequency auditory–nerve fibers in cat. J. Acoust. Soc. Am. 93:401–417, 1993.
Corwin, J. T., and J. C. Oberholtzer. Fish n' chicks: Model recipes for hair–cell regeneration? Neuron 19:951–954, 1997.
Dallos, P., R. Hallworth, and B. N. Evans. Theory of electrically driven shape changes of cochlear outer hair cells. J.Neurophysiol. 70:299–323, 1993.
de Boer, E. Mechanics of the cochlea: Modeling efforts. In: The Cochlea, edited by P. Dallos, A. N. Popper, and R. R. Fay. New York: Springer, 1996, pp. 258–317.
Deng, L., and C. D. Geisler. Responses of auditory–nerve fibers to nasal consonant—vowel syllables. J. Acoust. Soc. Am. 82:1977–1988, 1987.
Deng, L., C. D. Geisler, and S. Greenberg. Responses of auditory–nerve fibers to multitone complexes. J. Acoust. Soc. Am. 82:1989–2000, 1987.
Evans, E. F., and J. P. Wilson. The frequency selectivity of the cochlea. In: Basic Mechanisms in Hearing, edited by A. R. Møller. New York: Academic, 1973, pp. 519–554.
Geisler, C. D. From Sound to Synapse: Physiology of the Mammalian Ear. New York: Oxford, 1998.
Helmholtz, H. On the Sensations of Tone. New York: Dover, 1954.
Johnson, D. H. Point process models of single–neuron discharges. J. Comput. Neurosci. 3:275–299, 1996.
Johnson, D. H., and A. Swami. The transmission of signals by auditory–nerve fiber discharge patterns. J. Acoust. Soc. Am. 68:1115–1122, 1980.
Kros, C. J. Physiology of mammalian cochlear hair cells. In: The Cochlea, edited by P. Dallos, A. N. Popper, and R. R. Fay. New York: Springer, 1996, pp. 318–385.
Liberman, M. C. Single–neuron labeling and chronic cochlear pathology. I. Threshold shift and characteristic–frequency shift. Hear. Res. 16:33–41, 1984.
Liberman, M. C., and L. W. Dodds. Single–neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves. Hear. Res. 16:55–74, 1984.
Miller, R. L., J. R. Schilling, K. R. Franck, and E. D. Young. Effects of acoustic trauma on the representation of the vowel / e/ in cat auditory–nerve fibers. J. Acoust. Soc. Am. 101:3602–3616, 1997.
Miller, R. L., B. M. Calhoun, and E. D. Young. Contrast enhancement improves the representation of / e/–like vowels in the hearing–impaired auditory nerve. J. Acoust. Soc. Am. 106:2693–2708, 1999.
Molnar, C. E., and R. R. Pfeiffer. Interpretations of spontaneous discharge patterns of neurons in the cochlear nucleus. Proc. IEEE 56:993–1004, 1966.
Moore, B. C. J. Perceptual Consequences of Cochlear Damage. New York: Oxford, 1995.
Narayan, S. S., A. N. Temchin, A. Recio, and M. A. Ruggero. Frequency tuning of basilar membrane and auditory nerve fibers in the same cochleae. Science 282(5395):1882–1884, 1998.
NIH Consensus Statement. Noise Hear. Loss 8:1–24, 1990.
Nobili, R., F. Mammano, and J. Ashmore. How well do we understand the cochlea? TINS 21:159–167, 1998.
Patuzzi, R. B. Cochlear micromechanics and macromechanics. In: The Cochlea, edited by P. Dallos, A. N. Popper, and R. R. Fay. New York: Springer, 1996, pp. 186–257.
Peterson, G. E., and H. L. Barney. Control methods used in a study of the vowels. J. Acoust. Soc. Am. 24:175–184, 1952.
Pfeiffer, R. R. Classification of response patterns of actionpotential discharges for units in the cochlear nucleus: Tone–burst stimulation. Exp. Brain Res. 1:220–235, 1966.
Pols, L. C., L. J. van der Kamp, and R. Plomp. Perceptual and physical space of vowel sounds. J. Acoust. Soc. Am. 46:458–467, 1969.
Raphael, R. M., A. S. Popel, and W. E. Brownell. A membrane bending model of outer hair cell electromotility. Biophys. J. 78:2844–2862, 2000.
Robles, L., and M. A. Ruggero. Mechanics of the mammalian cochlea. Physiol. Rev. 81:1305–1352, 2001.
Rose, J. E., J. F. Brugge, D. J. Anderson, and J. E. Hind. Phase–locked response to low–frequency tones in single auditory–nerve fibers of the squirrel monkey. J. Neurophysiol. 30:769–793, 1967.
Ruggero, M. A., and N. C. Rich. Furosemide alters organ of Corti mechanics: Evidence for feedback of outer hair cells upon the basilar membrane. J. Neurosci. 11:1057–1067, 1991.
Sachs, M. B. Speech encoding in the auditory nerve. In: Hearing Science, Recent Advances, edited by C. I. Berlin, San Diego: College–Hill, 1984, pp. 263–307.
Schilling, J. R., R. L. Miller, M. B. Sachs, and E. D. Young. Frequency–shaped amplification changes the neural representation of speech with noise–induced hearing loss. Hear. Res. 117:57–70, 1998.
Sewell, W. F. Neurotransmitters and synaptic transmission. In: The Cochlea, edited by P. Dallos, A. N. Popper, and R. R. Fay. New York: Springer, 1996, pp. 503–553.
Siebert, W. M. Some implications of the stochastic behavior of primary auditory neurons. Kybernetik 2:206–215, 1965.
Smith, R. L., and J. J. Zwislocki. Responses of some neurons in the cochlear nucleus to tone–intensity increments. J. Acoust. Soc. Am. 50:1520–1525, 1971.
Spector, A. A., M. Ameen, and A. S. Popel. Simulation of motor–driven cochlear outer hair cell electromotility. Biophys. J. 81:11–24, 2001.
Stone, J. S., and E. W. Rubel. Cellular studies of auditory hair cell regeneration in birds. Proc. Natl. Acad. Sci. U.S.A. 97:11714–11721, 2000.
Wong, J. C., R. L. Miller, B. M. Calhoun, M. B. Sachs, and E. D. Young. Effects of high sound levels on responses to the vowel /eh/ in cat auditory nerve. Hear. Res. 123:61–77, 1998.
Young, E. D., and M. B. Sachs. Representation of steadystate vowels in the temporal aspects of the discharge patterns of populations of auditory–nerve fibers. J. Acoust. Soc. Am. 66:1381–1403, 1979.
Zhang, X., M. G. Heinz, I. C. Bruce, and L. H. Carney. A phenomenological model for the responses of auditory–nerve fibers: I. Nonlinear tuning with compression and suppression. J. Acoust. Soc. Am. 109:648–670, 2001.
Zheng, J., W. Shen, D. Z. He, K. B. Long, L. D. Madison, and P. Dallos. Prestin is the motor protein of cochlear outer hair cells. Nature (London) 405(6783):149–155, 2000.
Zheng, J. L., and W. Q. Gao. Overexpression of Math 1 induces robust production of extra hair cells in postnatal rat inner ears. Nat. Neurosci. 3:580–586, 2000.
Zweig, G., R. Lipes, and J. R. Pierce. The cochlear compromise. J. Acoust. Soc. Am. 59:975–982, 1976.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sachs, M.B., Bruce, I.C., Miller, R.L. et al. Biological Basis of Hearing-Aid Design. Annals of Biomedical Engineering 30, 157–168 (2002). https://doi.org/10.1114/1.1458592
Issue Date:
DOI: https://doi.org/10.1114/1.1458592