Abstract
The purpose of this work is the development of a robust and reliable three-dimensional (3D) Cartesian imaging technique for fast and flexible retrospective 4D abdominal MRI during free breathing. To this end, a non-uniform quasi random (NU-QR) reordering of the phase encoding (ky–kz) lines was incorporated into 3D Cartesian acquisition. The proposed sampling scheme allocates more phase encoding points near the k-space origin while reducing the sampling density in the outer part of the k-space. Respiratory self-gating in combination with SPIRiT-reconstruction is used for the reconstruction of abdominal data sets in different respiratory phases (4D-MRI). Six volunteers and three patients were examined at 1.5 T during free breathing. Additionally, data sets with conventional two-dimensional (2D) linear and 2D quasi random phase encoding order were acquired for the volunteers for comparison. A quantitative evaluation of image quality versus scan times (from 70 s to 626 s) for the given sampling schemes was obtained by calculating the normalized mutual information (NMI) for all volunteers. Motion estimation was accomplished by calculating the maximum derivative of a signal intensity profile of a transition (e.g. tumor or diaphragm).
The 2D non-uniform quasi-random distribution of phase encoding lines in Cartesian 3D MRI yields more efficient undersampling patterns for parallel imaging compared to conventional uniform quasi-random and linear sampling. Median NMI values of NU-QR sampling are the highest for all scan times. Therefore, within the same scan time 4D imaging could be performed with improved image quality.
The proposed method allows for the reconstruction of motion artifact reduced 4D data sets with isotropic spatial resolution of 2.1 × 2.1 × 2.1 mm3 in a short scan time, e.g. 10 respiratory phases in only 3 min. Cranio-caudal tumor displacements between 23 and 46 mm could be observed.
NU-QR sampling enables for stable 4D-MRI with high temporal and spatial resolution within short scan time for visualization of organ or tumor motion during free breathing. Further studies, e.g. the application of the method for radiotherapy planning are needed to investigate the clinical applicability and diagnostic value of the approach.
Export citation and abstract BibTeX RIS
1. Introduction
Two methods are mainly applied to produce 4D-MRI data sets in free-breathing: (i) real-time MRI using fast 3D sequences (Blackall et al 2006, Plathow et al 2006, Dinkel et al 2009) and (ii) data sorting using an internal or external respiratory surrogate (von Siebenthal et al 2007, Tokuda et al 2008, Cai et al 2011, Hu et al 2013, Tryggestad et al 2013, Yang et al 2015, Deng et al 2016, Han et al 2017, Küstner et al 2017, Rank et al 2017). For real-time MRI temporal and spatial resolution is limited by available hardware. Fast imaging sequences and the use of parallel imaging (PI) techniques allow the acquisition of 3D images with a temporal resolution of 1 s and typical voxel size of 4 mm. However, a typical human's breathing cycle is about 4–5 s making it impossible to acquire high-resolution 4D datasets without compromising image quality. Blackall et al (2006) proposed the use of dynamic 3D MRI to support radiotherapy planning. They achieved an acquisition time of 330 ms/image volume, but the image quality was insufficient to provide important structures and details. Dinkel et al (2009) used a time-resolved 3D FLASH sequence to investigate the complex breathing patterns in patients with hemidiaphragmatic paralysis due to malignant infiltration.
Most of the techniques of the second approach are based on multislice 2D k-space acquisitions under free-breathing with prospective (Tokuda et al 2008, Hu et al 2013) or retrospective (von Siebenthal et al 2007, Cai et al 2011, Tryggestad et al 2013, Paganelli et al 2015) gating and slice-reformatting to 4D datasets. Thus, this approach may suffer from reformatting artefacts for example due to imperfect slice profiles and low slice-resolution (5–10 mm).
To overcome these limitations techniques using free-breathing 3D measurements were used. Both, approaches based on radial (Buerger et al 2012, Chandarana et al 2013, Deng et al 2016) and Cartesian (Cheng et al 2015, Prieto et al 2015, Zhu et al 2016, Han et al 2017, Küstner et al 2017) sampling were presented.
Radial imaging exhibits advantageous motion reduction properties because of oversampling of the k-space center. However, radial approaches are often limited by gradient errors and off-resonance effects. Furthermore, non-Cartesian trajectories intrinsically have a lower signal-to noise ratio (SNR) and therefore reduced sampling efficiency compared to Cartesian imaging (Pipe and Duerk 1995, Lauzon and Rutt 1998). For this reason, many investigators have reverted to Cartesian sequences. Moreover, Cartesian imaging is still the standard in clinical routine examinations. Most of the proposed Cartesian-based methods reorder Cartesian k-space to radial (Zhu et al 2016) or spiral (Cheng et al 2015, Prieto et al 2015, Han et al 2017) trajectories. Recently, Küstner et al (2017) presented an approach based on pure pseudo random 3D Cartesian subsampling. External or internal respiratory signals are required to obtain motion-corrected free-breathing 3D images. External respiratory surrogates were typically acquired using breathing belts (Vedam et al 2002). However, belt signals are highly susceptible to hysteresis effects, drifts and representative of respiratory motion patterns of the chest wall rather than the direct respiratory motion patterns of the internal tissues of interest. The use of additional hardware can be avoided with internal retrospective self-gating using the k-space-center signal (DC signal) (Larson et al 2005, Weick et al 2012). The DC signal represents the total signal of the excitation volume. It reflects changes in the average signal due to volumetric changes in the abdomen which are induced by respiratory and cardiac motion and can be used as navigator signal. These changes can be recorded by phased-array coils due to their locally limited sensitivity. The recorded DC signal of the single coil elements is usually very stable over time and no drifts can be observed. To this end, the coil array has to be fixed well on the patient. Changes of the position of the coil element on the patient caused e.g. by coughing or sneezing can lead to DC signal perturbations which can be accounted for in the retrospective reconstruction process.
Retrospective data-sorting requires an increase in scan time as multiple fully encoded 3D datasets have to be acquired several times to compensate for rejected data within the gating process. Otherwise, undersampling artifacts have to be accepted due to not sampled k-space lines.
In recent years, compressed sensing (CS) (Lustig et al 2007) and PI techniques like generalized autocalibrating partially parallel acquisitions (GRAPPA) (Griswold et al 2002) and sensitivity encoding (SENSE) (Pruessman et al 1999) have been exploited to remove undersampling artefacts and consequently allow for an acceleration of 4D-MRI.
For CS and several PI methods incoherent random undersampling is required for successful and stable image reconstruction (Lustig et al 2007, Lustig and Pauly 2010). It was recently shown, that quasi-random (QR) sampling is beneficial to avoid synchronization between the repeated volume encoding and the periodic respiratory motion to obtain a uniform distribution of accepted and missing k-space lines after the gating process (Weick et al 2017). This can be achieved by reordering the phase and partition encoding steps (ky and kz) according to QR numbers (Zaremba 1968, Niederreiter 1978). QR numbers are deterministic and unlike pseudo-random numbers, a number in a QR series depends on information about all its predecessors, aiming at minimizing gaps in the covered range. This is desirable for sampling a k-space grid where both clustering and thinning out in arbitrary regions of the grid should be avoided. Without a priori knowledge about the total number of samples, a set of QR numbers covers a certain space more uniform than pseudo randomly distributed numbers and therefore leads to a more uniform sampled Cartesian k-space after the gating process. Nevertheless, missing lines near the k-space center can occur after the gating process resulting in coherent artifacts and potentially reduced performance of PI reconstruction.
The purpose of this work is to propose a 3D Cartesian sampling scheme leading to increased imaging efficiency, allowing for scan time reduction in 4D-MRI and simultaneously achieving high quality phase-resolved images. To this end, a non-uniform quasi random (NU-QR) sampling is employed that oversamples the k-space center in the expense of the uniform distribution. The acquisition scheme enables arbitrary retrospective data sorting with improved robustness of both, self-gating and PI reconstruction. It allows reconstruction of respiratory phase-resolved 3D images with isotropic spatial resolution and an arbitrary number of temporal breathing phases.
The feasibility of the approach to monitor breathing induced motion was demonstrated by abdominal imaging of six healthy volunteers and three patients with various abdominal lesions.
2. Materials and methods
2.1. Image acquisition
Conventional linear, uniform QR and the non-uniform QR (NU-QR) sampling pattern were implemented for 3D Cartesian imaging. 2D QR numbers were generated using a low-discrepancy sequence generator (Niederreiter 1978, Bratley et al 1992). Phase-encoding was then rearranged accordingly.
The NU-QR sampling pattern oversamples the k-space center according to a normal distribution. To this end, uniformly distributed quasi random numbers (X) were transformed to a normal distribution before mapping (Meyer et al 2015). The normal distribution (Y) was generated by inverse transform sampling according to:
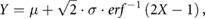
where µ is the mean value, σ the standard deviation and erf the error function. For computing the inverse error function an approximation of the inverse cumulative normal function was used.
The width of the distribution was set to the 2σ interval as a compromise between oversampling the center and sampling the periphery of k-space.
2.2. Retrospective data sorting
Retrospective respiratory self-gating using the DC signal was performed to reduce motion artifacts and to reconstruct abdominal images in multiple breathing states (Crowe et al 2004, Brau and Brittain 2006). The DC signal was analyzed for all single coil elements to get the predominantly respiratory motion information. Usually the coil element near the diaphragm detects the highest temporal variation in spin density and was selected manually for respiratory gating. A moving average filter was used to smooth high-frequency variations in the DC time course.
The continuously acquired k-space lines are sorted into the different gating windows allowing the reconstruction of 3D datasets in different respiratory phases (4D imaging).
The gating window range is defined by a sliding window approach. The window width w is calculated by
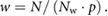
N is the total navigator range, Nw the total number of different gating windows and p is the pitch defining the size of the overlap: p = 1 means no overlap between the different gating windows, p > 1 that some data were completely ignored and p < 1 that some data were used several times for the reconstruction of adjacent respiratory states. Figure 1 illustrates the concept of data sorting using the DC signal derived from the continuously acquired data. Different threshold values in percentage of the individual global amplitude between end-expiration and end-inspiration define multiple different gating windows (amplitude binning). Consequently, the amount of data within one gating window varies based on the individual respiratory signals. Both, exhalation and inhalation data can be used for the reconstruction within the defined gating windows. Additionally, the derivation of the recorded DC signal can be used to distinguish between exhalation (negative derivation) and inhalation (positive derivation) within the same gating window (figure 1 solid and dashed line). The number of reconstructed respiratory phases Np equals Nw without and two times Nw with consideration of the DC signal derivation.
Figure 1. (A) Schematic illustration of data sorting. The colored boxes indicate different respiratory phases. The continuously acquired k-space lines are sorted according to these boxes using a sliding window approach. Positive derivation of the DC signal is associated with inspiratory phases (dashed) while negative derivation is associated with expiratory phases (solid). (B) End-expiratory (left) and end-inspiratory images (right). The dashed line shows the difference of the diaphragm position between expiratory and inspiratory phase.
Download figure:
Standard image High-resolution imageEnd-expiratory data sets were reconstructed using different pitch values for each volunteer to investigate the influence of the pitch value on image quality.
2.3. Image reconstruction
All images were reconstructed offline using MATLAB (MathWorks, Natick, Massachusetts) using a workstation with a 16-core Intel Xeon CPU and 125 GB RAM. Multiple accepted k-space lines were averaged. A GRAPPA based iterative parallel imaging reconstruction (SPIRiT) was applied after the gating process to reconstruct missing lines (Lustig and Pauly 2010). The temporal average of the time series with a kernel size of 5 × 5 × 5 was used for calibration. The reconstruction was disabled if at least more than three points right next to the central k-space point were missing after gating. After PI reconstruction, asymmetric readout was accounted for using an iterative projection onto convex sets (POCS) algorithm (Haacke et al 1991).
2.4. MR experiments
In vivo abdominal imaging during free breathing was performed on six healthy volunteers (age 31–60, 3 female, 3 male). Informed consent was obtained from each volunteer before measurement.
The imaging platform was a 1.5 T MAGNETOM Avanto scanner (Siemens Healthineers, Erlangen, Germany). A six-channel body array coil in combination with a six-channel spine array coil was used for signal reception. Data acquisition was performed with a 3D Cartesian FLASH pulse sequence, adapted to elliptical scanning and self-gating. Ten points of the DC signal were acquired and averaged within each excitation period (TR) after spatial encoding, by refocusing the moment of all imaging gradients (Weick et al 2012). Volunteer data was acquired with conventional linear, QR and NU-QR sampling with the motivation of a comparison of the different sampling schemes. Imaging parameters were chosen as follows: Field of view = 400 × 400 × 185 mm3, matrix size = 192 × 192 × 88, head-foot readout direction, repetition time/echo time = 4.0/1.2 ms, flip angle = 10°. A number of 10 repeated volumes were acquired in case of linear sampling for the volunteer measurements resulting in a total scan time of 11:36 min. The number of phase encoding steps for QR and NU-QR sampling were set to match the same total acquisition time.
Two male patients (aged 64 and 70 years) with liver metastasis and one female (aged 66) with an adrenal gland metastasis were referred to our department for stereotactic body radiotherapy. After written informed consent patients underwent measurement with NU-QR sampling. Identical imaging parameters with a reduced number of phase encoding steps resulting in a total acquisition time of 5:36 min were used.
Multiple respiratory motion states were generated retrospectively by sorting the continuously acquired data as described previously. The scan time was modified retrospectively for comparative analyses. To this end, a defined number of the first acquired k-space lines were discarded to create data sets with reduced numbers of lines. Thus the resulting datasets were equal to measurements with corresponding reduced scan times. Volunteer data sets were reconstructed with a pitch value of 0.3, ten gating windows (with/without considering the derivation of the DC signal, Np = 20/10) and reduced scan times of 7, 5 and 3 min. Patient data sets were reconstructed with a pitch value of 0.3 and Np = Nw = 10 using all acquired lines.
2.5. Quantitative data analysis
2.5.1. Assessment of pitch value influence and motion
A quantitative evaluation of the pitch value was performed by assessment of the coefficient of variation (CV) and the sharpness of the diaphragm in end-expiratory images. The sharpness of the transition describes the effectiveness of respiratory motion compensation while the CV value describes the influence on image quality in terms of SNR and inhomogeneity. Regions of interest (ROIs) were drawn in the liver and the CV calculated by dividing the standard deviation by the mean signal of the ROI. Signal intensity profiles along a drawn line perpendicular to the lung-liver interface were used to assess the sharpness. To this end, the maximum of first derivative (MD) of the signal intensity profile was computed. The larger the MD, the sharper the transition. An error of one pixel was assumed resulting from the calculation of the maximum derivation. Furthermore, the percentage of missing k-space lines (pML) after the gating process was compared for end-expiratory reconstructions for different pitch values.
The MD was also used to assess diaphragm and/or tumor expansion in cranio-caudal direction. To this end, the distance in millimeters D between the MD of the expiratory and inspiratory image was determined and used as a measurement of motion. The MD of the cranial transition in expiration and the MD of the caudal transition in inspiration was used to calculate tumor expansion. A moving average filter (5 points) was used to smooth out high frequency fluctuations. An exemplary determination of D is provided in figure 2.
Figure 2. Schematic illustration of motion assessment. Upper: signal intensity plot over structure (diaphragm transition or tumor). Lower: derivation of the profiles to assess distance D or total tumor range between inspiration and expiration.
Download figure:
Standard image High-resolution image2.5.2. Assessment of image quality
A quantitative evaluation of image quality versus acquisition time for the given sampling schemes was obtained by calculating the normalized mutual information (NMI) for all volunteers using the geometric mean of entropy (Strehl and Ghosh 2002, Pluim et al 2003) between an expiratory reference image data set and corresponding reduced scan time reconstructions. The central 60 slices (including most of the lung, liver and heart) which were cut in left-right direction were used for the calculation (matrix size 192 × 152 × 60). The reason for this was to exclude aliasing artifacts and consider incoherent undersampling artifacts in both phase encoding directions respectively. The reference data set was reconstructed for each sampling scheme by setting the gating window to only a few k-space lines being missed (max. 20) after the gating process. All acquired data were used for this reconstruction (no scan time reduction). The same gating window was then used for the reconstruction of time reduced data sets obtained as previously described.
3. Results
The results of the impact of various pitch values are shown in table 1. CV values of the ROIs, MD values of the transition as well as the percentage of missing lines (pML) averaged over all volunteers are presented. Increasing window width (decreasing the pitch) results in improved CV (the smaller the better) up to 30% and reduced undersampling ratio up to 94% from p = 1.0 to 0.2. In contrary, the lowest pitch value results in decreasing MD up to 43%. However, MD values do not increase within the standard deviation between p = 0.4 up to 1.0. The influence of different pitch values on image quality are visually depicted in figure 3.
Table 1. Mean and standard deviation of CV, MD as well as pML for various pitch values over all volunteers for end-expiratory phase.
| Pitch p | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 |
|---|---|---|---|---|---|---|---|---|---|
| CV (*10−2) | 5.1 ± 1.0 | 5.2 ± 0.8 | 5.7 ± 1.1 | 5.8 ± 1.3 | 6.2 ± 1.5 | 6.4 ± 1.2 | 6.5 ± 1.1 | 6.8 ± 1.4 | 7.3 ± 1.6 |
| MD | 0.9 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.2 | 1.4 ± 0.2 | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.1 |
| pML (%) | 2 ± 1 | 5 ± 3 | 9 ± 4 | 13 ± 5 | 18 ± 7 | 23 ± 9 | 28 ± 9 | 33 ± 9 | 37 ± 9 |
Figure 3. Images reconstructed with different pitch values of a volunteer dataset. Lower pitch values result in high signal intensity (low CV) but increased blurring (low MD) while higher pitch values result in decreased blurring (high MD) but lower signal intensity (high CV) and more prominent undersampling artifacts due to missing lines k-space lines (higher pML).
Download figure:
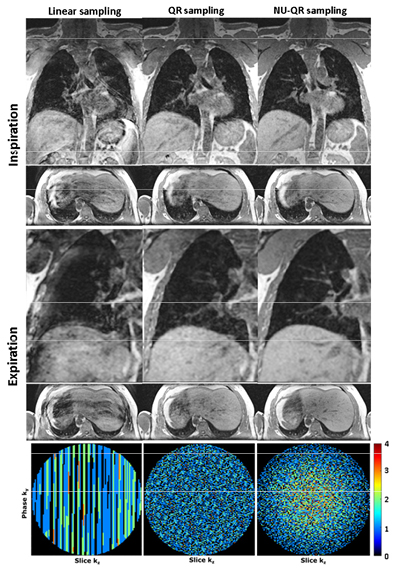
Standard image High-resolution imageThe comparison of different sampling schemes reconstructed for different scan times of 7, 5 and 3 min and Np = Nw = 10 breathing states can be seen in figure 4. Exemplary images of sagittal slices of a volunteer in end-expiratory and end-inspiratory are shown. Moreover, supporting videos V1 and V2 (stacks.iop.org/PMB/63/075002/mmedia) showing an exemplary respiratory motion-resolved coronal slice of data sets for a scan time of 3 min and Nw = 10 gating windows without (Np = 10) and with (Np = 20) consideration of the DC signal derivation. Additionally, figure 5 shows a detailed comparison of a coronal and transversal slice for a scan time of 3 min. Severe undersampling artifacts can be seen in case of linear sampling that strongly affects image quality. In contrast, reduced undersampling artifacts can be seen in case of QR and NU-QR sampling. However, QR sampling shows an increased noise level and incoherent artifacts compared to NU-QR sampling. Accordingly, small vessels in the liver and the lung can be differentiated more clearly in case of NU-QR sampling (see zoomed region). The corresponding distribution of sampled and missing phase encoding steps (kz and ky) after the gating process for the end-expiratory data set are shown in the lower row. Large areas of missing k-space lines can be seen for linear sampling while QR sampling leads to a uniform distribution. NU-QR sampling distributes more points near the k-space center and reduces the sampling density in the outer k-space.
Figure 4. Comparison of the proposed method with uniform linear and QR sampling of a volunteer dataset for different scan times. Expiratory and inspiratory images are shown in sagittal orientation.
Download figure:
Standard image High-resolution imageFigure 5. Comparison of the proposed method with uniform linear and QR sampling of a volunteer dataset for a scan time of 3 min. Expiratory and inspiratory images are shown in coronary and transversal orientation. Small vessels in the liver and lung can be differentiated more clearly in case of NU-QR sampling (zoomed region). Distribution of accepted and missing k-space lines after gating for the expiratory phase (lower row).
Download figure:
Standard image High-resolution imageNMI calculations are presented as boxplots for different scan times and sampling schemes in figure 6. The NMI decreases with increasing scan time reduction for all sampling schemes. Median NMI values of NU-QR sampling are the highest for all scan times respectively scan time reductions. NMI was not calculated for QR sampling with a scan time reduction of 90% because at least more than three points right next to the central k-space point were not accepted after gating for all volunteers. Figure 7 shows end-expiratory and end-inspiratory MR images in different orientations of the patient measurements as well as a corresponding expiratory slice of the radiotherapy treatment planning 4D-CT. Lesions can be more clearly differentiated on the MR images because of the superior soft-tissue contrast. Table 2 shows the results of the motion assessment. The displacement D of the diaphragm of the volunteers between end-expiratory and end-inspiratory phases is shown for scan times of 7 and 3 min respectively. The resulting values of the displacements of the two scan times are in agreement within the error limits for each volunteer. The range of motion in cranio-caudal direction of the lesions of the patients is displayed in the lower row.
Table 2. Displacement D of the diaphragm position of the volunteers for scan times of 3 and 7 min and range of motion expansion of tumors of the patients between end-expiration and end-inspiration. Patient 1 has three different lesions (highlighted in figure 7).
| Volunteers | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| D (mm) | ||||||
| 7 min | 23.1 ± 2.1 | 37.8 ± 2.1 | 25.2 ± 2.1 | 29.4 ± 2.1 | 37.8 ± 2.1 | 14.7 ± 2.1 |
| 3 min | 21.0 ± 2.1 | 37.8 ± 2.1 | 27.3 ± 2.1 | 31.5 ± 2.1 | 39.9 ± 2.1 | 12.6 ± 2.1 |
| Patients | 1 | 2 | 3 | |||
| Range of tumor motion (mm) | 46.2 ± 2.1 | |||||
| 35.7 ± 2.1 | 35.7 ± 2.1 | 31.5 ± 2.1 | ||||
| 23.1 ± 2.1 | ||||||
Figure 6. Boxplots of the NMI averaged over all volunteers versus scan time reductions respectively.
Download figure:
Standard image High-resolution imageFigure 7. Expiration and inspiration MR images of each patient acquired with NU-QR sampling. CT images are shown for comparison. Marked regions highlight the metastasis.
Download figure:
Standard image High-resolution image4. Discussion
The major limitation in 4D-MRI is the long scan time required to collect data in both, high spatial and high temporal resolution. The proposed method can capture a high number of motion states with isotropic spatial resolution of 2.1 × 2.1 × 2.1 mm3 in a short scan time, e.g. 10 respiratory phases in only 3 min.
Recently, it was shown that reordering of Cartesian k-space lines using QR numbers considerably improves image quality after respiratory self-gating (Weick et al 2017). The incoherent temporal and spatial ordering allows for a uniform spatial distribution over short periods of time without a priori knowledge about the lines which are accepted for image reconstruction. However, retrospective gating, especially at short acquisition times, may still produce missing lines in the k-space-center and thus coherent artefacts in the reconstructed image. This can easily be overcome by PI techniques as long as the gaps in k-space remain small enough to be correctly reconstructed by the parallel image reconstruction process. In this work a non-uniform QR sampling is presented to address these limitations. The proposed sampling scheme allocates more phase encoding points (ky, kz) near the k-space origin while reducing the sampling density in the outer part of the k-space (see figure 5 lower row). This results in oversampling of the k-space, thereby limiting the risk of missing lines near the center. A related strategy has recently been proposed for respiratory phase-resolved abdominal imaging using self-navigated 4D Cartesian imaging (Küstner et al 2017). However, the current approach uses a 2D QR k-space sampling. The incoherent temporal and spatial ordering of the QR sampling scheme allows for a more uniform spatial distribution after short periods of time compared to pseudo-random sampling. Whereas pseudo-random sampling results in clustering of points, QR schemes provide sampling that is more uniformly distributed and less clustered.
Because most of the energy of an image is concentrated near the k-space center at the low spatial frequencies, only less prominent and more incoherent (noise-like) aliasing artifacts from the low-energy outer k-space at high frequencies are obtained compared to a uniformly sampled k-space.
This can be observed, for example, in the better differentiation of vessels in liver and lung (figure 4). Improved motion artifact reduction can also be observed in radial imaging because the k-space-center is inherently oversampled. Thus, a detailed comparison of the proposed method and radial acquisitions regarding characterization and reduction of motion artifacts would be highly interesting. In a first step, the effect of different motion patterns on the point-spread-function (PSF) and on the noise characteristics (different averaging) have to be simulated for both acquisition strategies. Moreover, phantom and volunteer measurements have to be performed to verify the simulations.
A fast low-angle shot Gradient-Echo sequence combined with self-gating and non-uniform sampling is employed. The method enables for the determination of organ or tumor motion due to respiration in short scan times with high isotropic spatial resolution. The high flexibility due to the QR sampling allows for scan time reduction with simultaneous reconstruction of a high number of respiratory phases. These parameters can be chosen depending on the breathing-pattern to match a subject-specific reconstruction. To this end a trade-off between the desired temporal resolution, appropriate signal-to-noise-ratio, contrast-to-noise-ratio and artifact-level of the reconstructed images must be made.
Pitch values between 0.3 and 0.5 result in a good compromise between motion artifact reduction (MD) and signal intensity (CV). Lower pitch values resulted in increased blurring (18% between p = 0.3 and 0.2) and higher pitch values in decreased signal intensity (7% in CV and 38% in pML between p = 0.5 and 0.6). Depending on the situation (for example tumors are difficult to distinguish from the surrounding tissue or adjacent to organs at risk), different pitch values might be chosen. The NMI was used to compare the image quality between the samplings depending on scan time reductions. The median NMI values of NU-QR sampling are larger for all scan time reductions than those of linear as well as QR sampling. However, more subjects would be required to decide whether NU-QR is significantly better than QR sampling for all scan time reductions. Moreover, a threshold value could be defined, from which the image quality is supposed to be still diagnostically valuable. Missing lines had to be tolerated for some reference data sets because to large gating windows had to be used otherwise. Nevertheless, reference data sets had similar quality because only a few lines were placed in the center of k-space, whereas no lines were missed in the central grappa kernel region (reconstruction was disabled if more than three points were missed next to the central k-space point). The NMI calculation is sensitive to image artifacts and anatomical changes. This is minimized by using the same gating window for corresponding reconstructions of the time reduced data sets because anatomical changes can be neglected.
An important application of 4D-MRI is to monitor tumor movement and to determine tumor trajectories in radiation treatment planning. Volunteers and three patients with different diagnostic findings were examined in this study (figure 7). Motion artifact reduced 4D MRI data sets could be reconstructed successfully and tumor motion and/or diaphragm position between end-expiratory and end-inspiratory phases were quantitatively determined. The variation of the determined diaphragm position for 7 min and 3 min is within the assumed error limit of one pixel. Therefore the quality of the reconstruction with scan time of 3 min is supposed to be sufficient for motion assessment. Lesions in end-expiratory and end-inspiratory phase could be depicted more clearly compared to end-expiratory 4D CT, although the windowing was adjusted. A direct comparison of tumor motion was not possible because motion immobilization (belly press) was used for all 4D CT scans.
Even though the general condition of the patients was not good, data could be acquired without too much restriction because of the free-breathing acquisition. This method is more flexible even if the patient interrupts the measurement before data acquisition is completed. This is due to the fact that the k-space center is oversampled while the phase encoding steps are distributed quasi-randomly in k-space. Large gaps are minimized compared to conventional Cartesian sampling. This could also be useful in an MR-Linac setup where data can be acquired during radiation. Even for short treatment times, useful 4D MR data sets can be reconstructed retrospectively and the patient does not have to stay longer in the MR-Linac. It was demonstrated that the DC signal can be also used for cardiac gating (Brau et al 2006) and consequently the delineation of tumors close to the heart (figure 7 top row) could be further improved. However, cardiac gating would additionally reduce the amount of available data for reconstruction. The acquisition time that can be saved using the proposed sampling scheme (figures 4 and 5) could be invested in cardiac gating.
4D MRI data sets with 10 respiratory phases and an acquisition time of 3 min considering the derivation of the DC signal (differentiation of inspiratory and expiratory phases) were also reconstructed to demonstrate the potential of the proposed method. Severe undersampling artifacts can be seen in case of linear and QR sampling leading to diagnostically unusable reconstructions (supporting video V2). NU-QR sampling leads to much better suppression of undersampling artifacts allowing for diagnostically usable data sets. However, SNR is greatly reduced because only few data were used. The reconstruction of a full breathing cycle could be used to detect hysteresis effects within a breathing cycle (Seppenwoolde et al 2002) or to calculate 3D ventilation weighted data sets without administration of contrast agents using the SENCEFUL approach (Fischer et al 2014).
The proposed method could be integrated in the radiotherapy workflow at different steps. In the treatment planning process, 4D MRI can be acquired besides 4D CT to assist in the definition of patient specific target volumes of moving tumors especially when soft-tissue contrast is helpfully (figure 7). Amplitude binning was used for the 4D MRI reconstructions in this study because it was also used for 4D CT planning. However, comparing amplitude binning with other sorting algorithms (e.g. phase or cycle based) will be subject of future work. Furthermore, 4D MRI can be used during the treatment course to monitor response and to decide whether re-planning due to anatomical changes is necessary (without additional radiation exposure). In this study, the reconstruction time of the volunteer data sets (scan time = 5 min and Np = Nw = 10), was about 50 min on average. This is supposed to be fast enough for timely assessment and potential re-planning before the next fraction also for longer reconstruction times.
5. Conclusion
In this work a new method to further increase imaging efficiency of 4D respiratory free breathing MRI of the thorax and abdomen using Cartesian acquisition is shown. The method is of potential interest in clinical routine examinations because Cartesian imaging is still more accepted than radial imaging. It was shown that a trade-off between sampling uniformity and sampling frequency in the central k-space region further improves the robustness of the retrospective gating process. The proposed non-uniform QR sampling yields efficient undersampling patterns for PI after retrospective gating and therefore improves the imaging efficiency compared to uniform QR sampling. The proposed NU-QR sampling scheme was compared to sampling with linear and QR uniform distribution and was shown to achieve robust retrospective gating and improved image quality at identical scan times.
Acknowledgments
The authors have no relevant conflicts of interest.
Supplementary movie 1 (9.99 MB, avi) V1 4D 10phases 3min.
Supplementary movie 2 (8.00 KB, avi) V2 4D 20phases 3min.
Supplementary movie 3 (12.6 MB, avi) V3 patient1 10phases 5min.
Supplementary movie 4 (8.50 MB, avi) V4 patient2 10phases 5min 3min.
Supplementary movie 5 (4.14 MB, avi) V5 patient3 10phases 5min.