Abstract
We present an experimental verification of stopping-power-ratio (SPR) prediction from dual energy CT (DECT) with potential use for dose planning in proton and ion therapy. The approach is based on DECT images converted to electron density relative to water ϱe/ϱe, w and effective atomic number Zeff. To establish a parameterization of the I-value by Zeff, 71 tabulated tissue compositions were used. For the experimental assessment of the method we scanned 20 materials (tissue surrogates, polymers, aluminum, titanium) at 80/140Sn kVp and 100/140Sn kVp (Sn: additional tin filtration) and computed the ϱe/ϱe, w and Zeff with a purely image based algorithm. Thereby, we found that ϱe/ϱe, w (Zeff) could be determined with an accuracy of 0.4% (1.7%) for the tissue surrogates with known elemental compositions. SPRs were predicted from DECT images for all 20 materials using the presented approach and were compared to measured water-equivalent path lengths (closely related to SPR). For the tissue surrogates the presented DECT approach was found to predict the experimental values within 0.6%, for aluminum and titanium within an accuracy of 1.7% and 9.4% (from 16-bit reconstructed DECT images).
Export citation and abstract BibTeX RIS
1. Introduction
Radiotherapy with protons and heavier ions can provide superior target coverage due to an inverse depth dose profile with maximum dose deposition towards the finite ion range ('Bragg peak'). In order to fully exploit this asset of particle therapy, accurate stopping power prediction is necessary for the tissue traversed. Ideally one would use the treatment beam also for the imaging of the patient ('proton CT', 'ion CT'). Extensive research and promising results have been published in this field over the last years (Schulte et al 2005, Muraishi et al 2009, Telsemeyer et al 2012, Rinaldi et al 2013) showing a superior density resolution. First results of a real phantom imaged with a prototype proton CT scanner are reported in Vanzi et al (2013). Rit et al (2013) obtained an improved spatial resolution by accounting the most likely path of protons in the filtered back projection. Up to now, only research systems have been reported for stopping power CT measurements in phantoms and challenges concerning e.g. spatial resolution (proton CT) and dose levels (heavy ion CT) have to be solved still. Therewith, radiotherapy treatment planning for protons and heavier ions is still usually based on patients' x-ray CT data. Here, imaging and therapy involve very different types of radiation. Thus, photon attenuation of tissues represented by CT numbers have to be converted into stopping power ratios (SPRw) relative to water or water-equivalent path lengths (WEPL)6 for ions via a look-up table. State of the art for the look-up table compilation is the stoichiometric approach by Schneider et al (1996). The authors suggested scanning phantom materials with known chemical composition ('stoichiometry') to parameterize scanner (protocol) specific photon attenuation. The resultant dedicated scanner parameters can be used to derive virtual CT numbers of tabulated tissue compositions in the individual CT scanner (and protocols). Intrinsic translation errors originate since tissues and materials having the same photon attenuation can exhibit significantly different stopping powers for ions and vice versa.
More precise tissue information can be obtained through the availability of new imaging techniques and devices (PET-MRI, PET-CT, X nuclei MRI, MRI, phase contrast CT imaging, optical coherence tomography). Amongst these techniques, we investigate dual source CT imaging (Flohr et al 2006, Johnson et al 2007) which is already clinically available for its benefit in dose planning for particle therapy. By scanning an object with two different photon spectra Rutherford et al (1976), Torikoshi et al (2003), Bazalova et al (2008) and Landry et al (2011b) showed that a calculation of the electron density relative to water ϱe/ϱe, w and effective atomic number Zeff from dual energy CT (DECT) images is possible. However, most contributions in literature require the knowledge of the CT scanner spectra to derive the ϱe/ϱe, w and Zeff. In contrast, Krauss et al (2011) presented an approach to calculate the ϱe/ϱe, w purely imaged based and Saito (2012) showed that the calculation of the ϱe/ϱe, w is feasible by evaluating merely the CT number difference of both dual energy images. In a previous study we successfully correlated the ϱe/ϱe, w derived from DECT data by an image based approach to the SPRw (Hünemohr et al 2013).
In this contribution we investigate the ability of the DECT imaging technique to predict the SPRw more accurately by taking into account both the ϱe/ϱe, w and Zeff. With increasing differences in tissue compositions compared to water, the mean excitation value I influences significantly the stopping power as well as nuclear cross sections which depend on individual elemental tissue compositions. Andreo (2009) showed that particle ranges in clinically identical treated tissues can deviate just because of different elemental compositions and consequently I-values. He emphasized the need for the determination of individual tissue compositions along the complete beam path to reach sub-millimeter precision in particle therapy. Therefore, we considered tissue composition tables to estimate the I-value through the Zeff as originally discovered by Yang et al (2010). Through this approach, the SPRw can be calculated directly using the ϱe/ϱe, w and Zeff from DECT image data. In addition, knowledge on both ϱe/ϱe, w and Zeff allows more precise tissue segmentations for Monte Carlo simulations (Bazalova et al 2008, Landry et al 2011a) in Brachy and photon therapy.
The purpose of work is the experimental assessment of accuracy of our SPRw prediction from DECT data. Twenty materials (tissue surrogates, polymers, aluminum, titanium) were measured in a dual source CT scanner. The resulting ϱe/ϱe, w and Zeff images of the individual samples were compared to the corresponding reference values. Finally, SPRw predictions derived from the measured ϱe/ϱe, w and Zeff images (and the relation between the I-value and Zeff) were compared to carbon ion WEPL measurements for all materials.
2. Materials and methods
2.1. Prediction of the stopping power ratio from dual energy CT image data
Stopping power of swift ions is described by the Bethe equation. In Schneider et al (1996) the SPRw of a medium relative to water was approximated by the following ratio without higher orders:

The energy dependence of the SPR is considered very small (below 0.4%, Yang et al 2012) and is ignored by most analytical dose planning systems (e.g. there is only one Hounsfield look-up table which converts the entire CT image in WEPL). In this work the projectile energy was assumed to be 200 MeV/nucleon in accordance to experimental conditions. The I-value of water Iw was assumed to be 75 eV (ICRU 1993).
To predict the SPRw of a medium for a specific ion energy, the ϱe/ϱe, w and the I-value of the medium has to be known (equation (1)). With DECT data the ϱe/ϱe, w of the medium can be derived directly. The I-value of the medium has to be parameterized through the Zeff image in agreement with Yang et al (2010) by

The parameters a and b have to be defined in advance for an I-value prediction from measured Zeff image data. Therefore, tabulated elemental compositions of 71 standard human tissues were taken from Woodard and White (1986) and White et al (1987) to calculate reference Zeffs and reference I-values. The same tissues were used in Schneider et al (2000). For Zeff (equation (21)) the mass weight fractions were multiplied by the individual Zi/Ai ratio of each element to obtain the electron density weight ( ). I-values of the individual constituents were taken from ICRU (1993), table 2.11 and Bragg's additivity rule was applied for compounds to calculate the I-values. Thyroid was excluded because of its iodine amount which provokes a higher Zeff compared to tissues in that range with 'usual' compositions.
). I-values of the individual constituents were taken from ICRU (1993), table 2.11 and Bragg's additivity rule was applied for compounds to calculate the I-values. Thyroid was excluded because of its iodine amount which provokes a higher Zeff compared to tissues in that range with 'usual' compositions.
The 71 calculated reference Zeff and I-values were used for the linear parameterization and determination of a and b.
In summary the SPRw could be subsequently predicted from the DECT ϱe/ϱe, w and Zeff images by
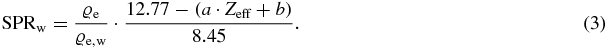
Through this equation it is clear that with DECT an almost direct imaging of SPRw is possible. The ϱe/ϱe, w exhibits the main target contribution to the SPRw for ions, the linearly parameterized I-value exhibits only a secondary order effect on predicted SPRw due to the logarithmic term (equation (1)).
2.2. DECT image based calculation of the ϱe/ϱe, w and Zeff
The x-ray attenuation spectrum for all body materials can be decomposed into a virtual pure photoelectric absorption material and a virtual pure Compton effect material:

where n is approximately equal to 3, while f(E) is an almost flat function of the photon energy and a1 and a2 are proportionality factors that are approximately independent of the material and can be obtained from measured data or theoretical calculations. They characterize the relative strengths of the Compton effect and the photoelectric effect, respectively (Alvarez and Macovski 1976).
In order to relate this equation to the CT-value, it is useful to define the weighted average 〈μ(E)〉, which depends on the detected x-ray energy spectrum S(E) as

For a thin absorber inside a water phantom of arbitrary size it is known that the commonly used CT-value x is related to this average attenuation coefficient of the absorber 〈μ(E)〉 and the average attenuation coefficient of water 〈μw(E)〉 by (Brooks 1977)

and this can be solved for 〈μ(E)〉:
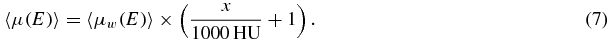
In order to extract the electron density, two spectra are used as indicated by the indices 1 and 2:
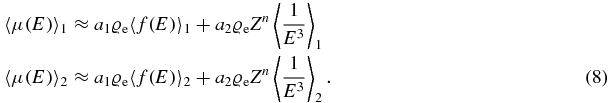
The first term depends on electron density, while the second term depends on electron density times some power of the atomic number. In order to get the electron density, we solve for the first term:
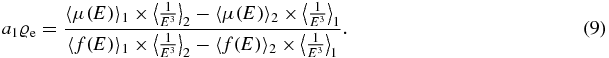
To simplify this, two constants b1 and b2 can be introduced, which only depend on the spectrum, but not on the studied material:

And after using the definition of the CT-value
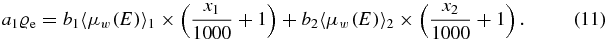
Dividing by a1ϱe, w leads to
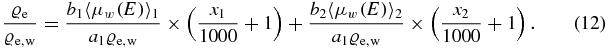
Now a new constant ce can be introduced:

By definition the CT-value of water is zero, which means that ce is the only unknown parameter and the electron density relative to water can be written as
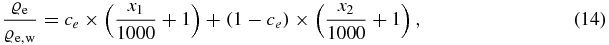
where ce depends on the two employed effective spectra, while x1 and x2 denote the two CT-values in Hounsfield units, respectively. A single calibration material, which is scanned in a single Dual energy scan, is therefore sufficient to determine the parameter ce. This is more practical than the fundamental approach to start from measured x-ray spectra. In the same way the effective atomic number can be determined by eliminating the first term in equation (4):
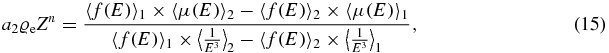
so that in a more compact notation using the abbreviations c1 and c2 we obtain:

Dividing by a2ϱe and inserting the definition of the Hounsfield unit results in
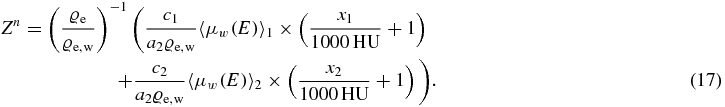
Now a new variable de can be introduced as:

Applying the equation to water shows that de is the only unknown parameter and hence the effective atomic number can be obtained from:
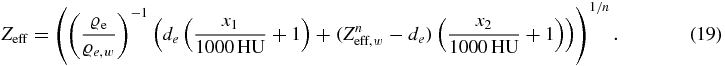
This procedure automatically fixes the equation to calculate the effective atomic number. In order to have the same photoelectric absorption for a mixture of atoms as for a hypothetical material with the same electron density and a certain effective atomic number, the constrain is

where ϱe, i denotes the contribution of atom type i to the total electron density. This can be rewritten in terms of the number density ni of atoms of type i, i.e. the number of atoms per unit volume of type i, where ϱe, i = ni × Zi:

and this definition is also used to calculate the effective atomic number in the equation above.
It is especially important that the same coefficient n shows up in equations (19) and (21) and that ni is the number density and not the mass density of the atom i. This difference matters for all hydrogen rich materials. The coefficient n has been determined by x-ray absorption simulations which indicate that for the range Z = 1, ..., 20 and the used CT spectra the optimum coefficient is n = 3.1. There are alternative definitions of the effective atomic number, which vary depending on the considered attenuation processes as well as on the studied materials. It should be noted that this method for calculating the electron density and effective atomic number from clinical CT-images is not applicable for high Z materials above iodine as the K-edge of these material will move into the low energy spectrum and thus invalidate equation (4). For our study we have chosen a single reference material for all measurements and we have taken the effect of beam-hardening into account, by correcting for the dependence of the measured CT-values of the calibration sample on the patient diameter. In addition a low pass filter (2D boxcar) was applied on both CT HU images to suppress noise.
2.3. Test materials and DECT scans
For this study different materials were measured in a DECT scanner to
- assess the imaged based calculation of ϱe/ϱe, w (previous section) and Zeff and compare them to reference data
- predict SPRw from ϱe/ϱe, w and Zeff images (2.1) and compare them to measured WEPL.
2.3.1. Measurements of various materials in a dual energy CT scanner
Material selection comprehends tissue surrogates (manufacturer Gammex RMI Middleton), various polymers, pure aluminum and titanium which were scanned in a second generation dual source CT scanner (Siemens Somatom Definition Flash). Table 1 lists reference properties of the measured materials. Specific elemental compositions were only available for the tissue surrogates (provided non-batch specific by the manufacturer) and both pure metals. For the polymers compositions had to be estimated and might deviate from actual values.
Table 1. Reference values for measured materials, elemental compositions by weight. Compositions for Gammex tissue surrogates were provided by the manufacturer non-batch specific for our set of cylinders. Compositions and I-values for PMMA and Teflon were taken from Berger et al (2005) as an estimation. For the other polymers composition was estimated. Reference Zeff and I-values are calculated according to equation (21) and Braggs' additivity rule (ICRU 1993). Metals had a purity of >99.9%. The relative electron densities to water for the Gammex tissue surrogates were provided batch-specific for each cylinder. For the other materials, the electron density was calculated from the measured mass density and the composition. WEPL (SPRw respectively) measurements were carried out with carbon ions of 200 MeV/u for tissue surrogates and PMMA (271 MeV/u for metal and other polymer probes) and material slabs of 1 cm thickness, for the measurement method see Jäkel (2001).
| Material | Class | Density (g cm−3) | ϱe, w | WEPL | Zeff | I (eV) | H | C | N | O | F | Mg | Al | Si | P | Cl | Ca | Ti |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung | Gammex | 0.460 | 0.444 | 0.444 | 7.46 | 73.78 | 8.47 | 59.57 | 1.97 | 18.11 | 0.00 | 11.21 | 0.00 | 0.58 | 0.00 | 0.10 | 0.00 | 0.00 |
| Adipose | Gammex | 0.942 | 0.925 | 0.943 | 6.17 | 66.56 | 9.06 | 72.30 | 2.25 | 16.27 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.13 | 0.00 | 0.00 |
| Breast | Gammex | 0.988 | 0.965 | 0.983 | 6.81 | 68.19 | 8.59 | 70.11 | 2.33 | 17.90 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.13 | 0.95 | 0.00 |
| True water | Gammex | 1.000 | 1.000 | 1.000 | 7.45 | 75.00 | 11.19 | 0.00 | 0.00 | 88.81 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Solid water | Gammex | 1.018 | 0.989 | 1.001 | 7.55 | 70.41 | 8.00 | 67.30 | 2.39 | 19.87 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.14 | 2.31 | 0.00 |
| Muscle | Gammex | 1.049 | 1.019 | 1.033 | 7.55 | 70.23 | 8.10 | 67.17 | 2.42 | 19.85 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.14 | 2.32 | 0.00 |
| Brain | Gammex | 1.052 | 1.048 | 1.064 | 6.05 | 63.54 | 10.83 | 72.54 | 1.69 | 14.86 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.08 | 0.00 | 0.00 |
| Liver | Gammex | 1.089 | 1.058 | 1.073 | 7.55 | 70.33 | 8.06 | 67.01 | 2.47 | 20.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.14 | 2.31 | 0.00 |
| Inner bone | Gammex | 1.147 | 1.099 | 1.099 | 10.14 | 80.09 | 6.67 | 55.64 | 1.96 | 23.52 | 0.00 | 0.00 | 0.00 | 0.00 | 3.23 | 0.11 | 8.86 | 0.00 |
| B200 | Gammex | 1.153 | 1.105 | 1.108 | 10.15 | 80.18 | 6.65 | 55.52 | 1.98 | 23.64 | 0.00 | 0.00 | 0.00 | 0.00 | 3.24 | 0.11 | 8.87 | 0.00 |
| CB30 | Gammex | 1.333 | 1.278 | 1.263 | 10.61 | 80.75 | 6.68 | 53.48 | 2.12 | 25.61 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.11 | 12.01 | 0.00 |
| CB50 | Gammex | 1.560 | 1.470 | 1.426 | 12.26 | 93.17 | 4.77 | 41.63 | 1.52 | 32.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.08 | 20.02 | 0.00 |
| Cortical bone | Gammex | 1.823 | 1.695 | 1.612 | 13.38 | 104.54 | 3.41 | 31.41 | 1.84 | 36.50 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 26.81 | 0.00 |
| Tecapeek | Polymer | 1.305 | 1.230 | 1.241 | 6.32 | 74.70 | 4.76 | 76.19 | 0.00 | 19.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Tecaform | Polymer | 1.410 | 1.353 | 1.354 | 6.98 | 77.50 | 6.67 | 40.00 | 0.00 | 53.33 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Tecadur | Polymer | 1.463 | 1.364 | 1.315 | 6.79 | 81.05 | 3.61 | 57.83 | 0.00 | 38.55 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| PMMA | Polymer | 1.183 | 1.149 | 1.165 | 6.50 | 74.00 | 8.05 | 59.98 | 0.00 | 31.96 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Teflon | Polymer | 2.151 | 1.860 | 1.782 | 8.45 | 99.10 | 0.00 | 24.02 | 0.00 | 0.00 | 75.98 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Aluminum | Metal | 2.699 | 2.343 | 2.140 | 13.00 | 166.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Titanium | Metal | 4.540 | 3.759 | 3.254 | 22.00 | 233.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 100.00 |
All materials were in a cylindrical form with a radius of r = 1.4 cm (tissue surrogates, polymers) and r = 0.75 cm for both metal probes. Materials were placed along the central scanner axis in a cylindrical PMMA tube (r = 8 cm) avoiding crosstalk and influences because of different positioning with respect to the isocenter (Hünemohr et al 2013).
Dual energy voltage pairs were set to 80/140Sn kVp and 100/140Sn kV with additional tin filtration of the higher spectrum. Tube currents were automatically set by the system to obtaining the same image quality from both tubes (pitch = 0.6; 80/140Sn kVp: 300/116 mAs, CTDIvol = 20.6 mGy; 100/140Sn kV: 300/232 mAs, CTDIvol = 42.5 mGy). A dedicated dual energy kernel (D30) was used to obtain quantitative CT-images without edge enhancement. For aluminum and titanium only a single rotation was measured and a similar but not commercially available quantitative kernel was applied and includes a raw data based DECT beam hardening correction. For both metals the reconstruction comprehends a 16-bit Hounsfield unit scale.
2.3.2. Accuracy of derived electron density and effective atomic number
The ϱe/ϱe, w and Zeff images were calculated from both DECT images with the algorithm presented in 2.2. Mean image values of CT number, ϱe/ϱe, w and Zeff of an inner cylinder of each material cylinder were evaluated. For both metals, mean values in a circular region-of-interest (ROI) of only one 16-bit reconstructed image slice were evaluated.
2.3.3. Accuracy of stopping-power-ratio prediction from DECT images
The SPRw for each measured material was predicted by taking the mean measured ϱe/ϱe, w and the I-value (estimated by the mean measured Zeff, equation (19) and the relation established in 2.1, equation (3)). The SPRw predictions based on DECT images were compared to measurements of WEPL with carbon ions performed with a variable water column ('peakfinder', PTW Freiburg) as described in Jäkel (2001). Tissue surrogates and PMMA were measured at 200 MeV/nucleon, the metals and other polymers at 271 MeV/nucleon.
3. Results and discussion
3.1. Parameterization of the I-value by the effective atomic number
Figure 1 shows the I-value parameterization through the Zeff from the 71 tabulated tissue compositions. Two distinct regions were observed (soft and bony tissue) and the SPRw was predicted from the DECT ϱe/ϱe, w and Zeff with equation (3) as follows:
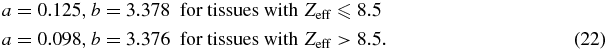
The reason for the division in two distinct regions is the difference of the effective to the mean atomic number (〈Z〉 = ∑iwiZi). Zeff represents a photon interaction quantity and is—due to the elevated photo electric interaction with high Z materials—weighted exponentially with n = 3.1 (equation (21),  ). Bony tissues containing calcium and phosphorus have a significant higher Zeff than 〈Z〉 since the high atomic Z constituents are weighted higher. Hence, the slope of lnI to Zeff is smaller for bony than for soft tissue due to the rapid increase of Zeff compared to 〈Z〉 with the I-value.
). Bony tissues containing calcium and phosphorus have a significant higher Zeff than 〈Z〉 since the high atomic Z constituents are weighted higher. Hence, the slope of lnI to Zeff is smaller for bony than for soft tissue due to the rapid increase of Zeff compared to 〈Z〉 with the I-value.
Figure 1. Ln I to Zeff fits of 71 tabulated tissues. Also data of Gammex surrogates, polymers and two measured metals (aluminum and titanium) are given as additional information. For the parameterization only the tabulated tissue compositions were considered. Note: materials having the same Zeff do not express necessarily the same I-value (e.g. aluminum), since the I-value depends on the specific type of bonding and atomic shell structure.
Download figure:
Standard image High-resolution imageParameters a and b derived from the linear fitting with tabulated tissue compositions were then applied to the measured Zeff images for the I-value prediction.
Here, I-value residuals for the investigated measured tissue surrogates (table 3) emerge from the I-value parameterization through the Zeff which was conducted with 71 tabulated tissues compositions (figure 1). As mentioned in Hünemohr et al (2013) tissue surrogates have a different composition compared to real tissue, especially the carbon and oxygen are interchanged in the epoxy bases surrogates.
3.2. Accuracy of the electron density and effective atomic number
Measured mean values of ϱe/ϱe, w and Zeff derived from both DECT images are given in table 2 along with mean CT numbers for both image acquisitions (80/140Sn kVp and 100/140Sn kVp). For the measured tissue surrogates the ϱe/ϱe, w was determined with mean accuracy of 0.4% from the DECT images of both voltage pairs. An overall mean accuracy of 1.7% (1.9%) for the Zeff was achieved with the 80/140Sn kVp (100/140Sn kVp) DECT images of the tissue surrogate (excluding the lung surrogate). Here an absolute mean accuracy of 0.14 (0.15) is obtained for the Zeff at 80/140Sn kVp (100/140Sn kVp). A Zeff calculation for the lung material was not possible due to the instability caused by the division by the low ϱe/ϱe, w (equation (19)). We notice a higher difference (outside the 2-σ range) between the measured and the reference Zeff of the brain surrogate (6%) which has been observed also by Goodsitt et al (2011). The tissue surrogates used in this article are part of the Gammex electron density CT phantom providing high precision in the reference ϱe/ϱe, w of the materials (batch specific), whereas less control of the material composition (and hence Zeff) in the production process might be a possible explanation for the higher Zeff residuals.
Table 2. Measured mean values of CT numbers, ϱe and Zeff dependent on two different dual energy tube voltage pairs.
| Voltage pair | Material | Class | Pixels | HUlow kVp | HUhigh kVp |  |
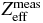 |
|---|---|---|---|---|---|---|---|
| 80 140 | Lung | Gammex | 31 331 | −556 ± 27 | −559 ± 28 | 0.442 ± 0.027 | NA |
| 100 140 | Lung | Gammex | 31 331 | −559 ± 27 | −560 ± 27 | 0.441 ± 0.027 | NA |
| 80 140 | Adipose | Gammex | 39 576 | −124 ± 8 | −85 ± 8 | 0.932 ± 0.005 | 6.30 ± 0.20 |
| 100 140 | Adipose | Gammex | 39 576 | −109 ± 5 | −85 ± 6 | 0.931 ± 0.004 | 6.33 ± 0.18 |
| 80 140 | Breast | Gammex | 37 927 | −59 ± 8 | −39 ± 9 | 0.970 ± 0.005 | 6.91 ± 0.16 |
| 100 140 | Breast | Gammex | 37 927 | −51 ± 5 | −39 ± 6 | 0.970 ± 0.004 | 6.93 ± 0.14 |
| 80 140 | True water | Gammex | 18 139 | −2 ± 8 | −1 ± 9 | 1.000 ± 0.005 | 7.40 ± 0.14 |
| 100 140 | True water | Gammex | 18 139 | 0 ± 5 | 0 ± 6 | 1.000 ± 0.004 | 7.44 ± 0.12 |
| 80 140 | Solid water | Gammex | 28 033 | 8 ± 8 | −1 ± 9 | 0.994 ± 0.006 | 7.67 ± 0.13 |
| 100 140 | Solid water | Gammex | 28 033 | 5 ± 6 | −1 ± 6 | 0.994 ± 0.004 | 7.69 ± 0.11 |
| 80 140 | Muscle | Gammex | 39 576 | 39 ± 9 | 29 ± 10 | 1.025 ± 0.006 | 7.67 ± 0.13 |
| 100 140 | Muscle | Gammex | 39 576 | 36 ± 7 | 29 ± 7 | 1.024 ± 0.005 | 7.69 ± 0.12 |
| 80 140 | Brain | Gammex | 39 576 | −8 ± 8 | 32 ± 9 | 1.049 ± 0.005 | 6.41 ± 0.17 |
| 100 140 | Brain | Gammex | 39 576 | 7 ± 5 | 32 ± 6 | 1.048 ± 0.004 | 6.44 ± 0.16 |
| 80 140 | Liver | Gammex | 39 576 | 78 ± 8 | 69 ± 9 | 1.065 ± 0.006 | 7.65 ± 0.12 |
| 100 140 | Liver | Gammex | 39 576 | 76 ± 6 | 68 ± 7 | 1.063 ± 0.004 | 7.69 ± 0.11 |
| 80 140 | Inner bone | Gammex | 39 576 | 351 ± 10 | 176 ± 9 | 1.098 ± 0.006 | 10.11 ± 0.09 |
| 100 140 | Inner bone | Gammex | 39 576 | 286 ± 7 | 176 ± 7 | 1.097 ± 0.004 | 10.13 ± 0.08 |
| 80 140 | B200 | Gammex | 37 927 | 360 ± 10 | 185 ± 10 | 1.107 ± 0.006 | 10.08 ± 0.09 |
| 100 140 | B200 | Gammex | 37 927 | 295 ± 7 | 185 ± 7 | 1.106 ± 0.005 | 10.10 ± 0.08 |
| 80 140 | CB30 | Gammex | 39 576 | 657 ± 11 | 389 ± 10 | 1.269 ± 0.007 | 10.73 ± 0.08 |
| 100 140 | CB30 | Gammex | 39 576 | 557 ± 8 | 389 ± 7 | 1.269 ± 0.005 | 10.74 ± 0.07 |
| 80 140 | CB50 | Gammex | 37 927 | 1212 ± 14 | 695 ± 11 | 1.463 ± 0.008 | 12.16 ± 0.08 |
| 100 140 | CB50 | Gammex | 39 576 | 1014 ± 9 | 694 ± 8 | 1.463 ± 0.006 | 12.14 ± 0.07 |
| 80 140 | Cortical bone | Gammex | 39 576 | 1823 ± 21 | 1045 ± 12 | 1.696 ± 0.009 | 13.05 ± 0.08 |
| 100 140 | Cortical bone | Gammex | 41 225 | 1515 ± 13 | 1044 ± 9 | 1.700 ± 0.008 | 12.97 ± 0.07 |
| 80 140 | Tecapeek | Polymer | 28 033 | 160 ± 10 | 210 ± 12 | 1.232 ± 0.008 | 6.32 ± 0.21 |
| 100 140 | Tecapeek | Polymer | 29 682 | 180 ± 7 | 209 ± 9 | 1.230 ± 0.006 | 6.41 ± 0.18 |
| 80 140 | Tecaform | Polymer | 28 033 | 324 ± 12 | 350 ± 13 | 1.362 ± 0.008 | 6.94 ± 0.19 |
| 100 140 | Tecaform | Polymer | 29 682 | 336 ± 8 | 350 ± 9 | 1.361 ± 0.007 | 6.99 ± 0.21 |
| 80 140 | Tecadur | Polymer | 29 682 | 595 ± 20 | 432 ± 17 | 1.359 ± 0.012 | 9.59 ± 0.11 |
| 100 140 | Tecadur | Polymer | 28 033 | 536 ± 16 | 432 ± 15 | 1.358 ± 0.012 | 9.63 ± 0.09 |
| 80 140 | PMMA | Polymer | 28 033 | 100 ± 11 | 139 ± 12 | 1.157 ± 0.007 | 6.52 ± 0.21 |
| 100 140 | PMMA | Polymer | 28 033 | 116 ± 7 | 140 ± 8 | 1.157 ± 0.005 | 6.56 ± 0.18 |
| 80 140 | Teflon | Polymer | 29 682 | 972 ± 15 | 899 ± 15 | 1.865 ± 0.011 | 8.29 ± 0.13 |
| 100 140 | Teflon | Polymer | 26 384 | 949 ± 10 | 900 ± 10 | 1.865 ± 0.008 | 8.35 ± 0.13 |
| 80 140 | Aluminum | Metal | 193 | 2751 ± 20 | 1761 ± 13 | 2.280 ± 0.016 | 13.55 ± 0.05 |
| 100 140 | Aluminum | Metal | 193 | 2365 ± 15 | 1764 ± 11 | 2.285 ± 0.013 | 13.62 ± 0.04 |
| 80 140 | Titanium | Metal | 193 | 12338 ± 166 | 6011 ± 20 | 3.438 ± 0.069 | 22.99 ± 0.33 |
| 100 140 | Titanium | Metal | 193 | 9530 ± 100 | 6029 ± 22 | 3.496 ± 0.017 | 23.11 ± 0.16 |
Calculations of the aluminum and titanium material suffer from scattering provoked by the elevated photon attenuation of the high Z materials. Figure 2 shows the individual ϱe/ϱe, w and Zeff residuals for each tissue surrogate compared to reference values (table 1). Table 3 lists all results in absolute and percentage residuals to reference values.
Figure 2. Residuals for measured tissue surrogates with elemental composition provided by the manufacturer. Zeff could not be calculated for lung and hence also the I-value could not be estimated. For the SPRw prediction of lung, only the ϱe/ϱe, w was taken into account. SPRws were calculated for a projectile energy of 200 MeV/u.
Download figure:
Standard image High-resolution imageTable 3. Absolute and percentage residuals of measured ϱe and Zeff, predicted SPRw and I compared to reference values (table 1) For the tissue surrogates the reference composition (hence Zeff) might not be the true compositions since the manufacturer gives only an estimate of non-batch specific compositions. Also compositions of the polymers were estimated from literature values. The high discrepancy in Zeff of Tecadur might result from an additional high Z material in low concentration (e.g. antimony) involved in the production process. For lung tissue a calculation of Zeff was not possible and therewith also the I estimation. SPRws were calculated for a projectile energy of 200 MeV/u.
| Voltage pair | Material | Class | SPRw res. (%) | ϱe res. (%) | Zeff res. (%) | I res. (%) | SPRw res. abs. | ϱe res. abs. | Zeff res. abs. | I res. abs. (eV) |
|---|---|---|---|---|---|---|---|---|---|---|
| 80 140 | Lung | Gammex | 0.5 | 0.5 | – | – | 0.002 | 0.002 | NA | NA |
| 100 140 | Lung | Gammex | 0.7 | 0.7 | – | – | 0.003 | 0.003 | NA | NA |
| 80 140 | Adipose | Gammex | 0.6 | 0.8 | 2.1 | 3.2 | 0.006 | 0.007 | 0.13 | 2.16 |
| 100 140 | Adipose | Gammex | 0.5 | 0.6 | 2.6 | 2.9 | 0.005 | 0.006 | 0.16 | 1.92 |
| 80 140 | Breast | Gammex | 0.4 | 0.5 | 1.5 | 1.9 | 0.004 | 0.005 | 0.10 | 1.30 |
| 100 140 | Breast | Gammex | 0.4 | 0.5 | 1.8 | 2.2 | 0.004 | 0.005 | 0.12 | 1.47 |
| 80 140 | True water | Gammex | 0.2 | 0.0 | 0.6 | 1.5 | 0.002 | 0.000 | 0.04 | 1.13 |
| 100 140 | True water | Gammex | 0.2 | 0.0 | 0.1 | 1.0 | 0.002 | 0.000 | 0.00 | 0.76 |
| 80 140 | Solid water | Gammex | 0.9 | 0.5 | 1.7 | 8.5 | 0.009 | 0.005 | 0.12 | 5.99 |
| 100 140 | Solid water | Gammex | 0.9 | 0.5 | 1.9 | 8.8 | 0.009 | 0.005 | 0.15 | 6.18 |
| 80 140 | Muscle | Gammex | 1.0 | 0.6 | 1.6 | 8.8 | 0.010 | 0.006 | 0.12 | 6.17 |
| 100 140 | Muscle | Gammex | 1.1 | 0.5 | 1.9 | 9.1 | 0.011 | 0.005 | 0.14 | 6.36 |
| 80 140 | Brain | Gammex | 0.3 | 0.1 | 6.0 | 2.8 | 0.003 | 0.001 | 0.36 | 1.75 |
| 100 140 | Brain | Gammex | 0.1 | 0.0 | 6.4 | 3.1 | 0.001 | 0.000 | 0.39 | 1.99 |
| 80 140 | Liver | Gammex | 0.9 | 0.7 | 1.4 | 8.4 | 0.010 | 0.007 | 0.10 | 5.88 |
| 100 140 | Liver | Gammex | 1.1 | 0.5 | 1.9 | 8.9 | 0.012 | 0.005 | 0.14 | 6.26 |
| 80 140 | Inner bone | Gammex | 0.6 | 0.1 | 0.3 | 1.5 | 0.007 | 0.001 | 0.03 | 1.17 |
| 100 140 | Inner bone | Gammex | 0.8 | 0.2 | 0.1 | 1.3 | 0.009 | 0.002 | 0.01 | 1.02 |
| 80 140 | B200 | Gammex | 0.6 | 0.2 | 0.7 | 1.9 | 0.007 | 0.002 | 0.07 | 1.50 |
| 100 140 | B200 | Gammex | 0.7 | 0.1 | 0.5 | 1.7 | 0.008 | 0.001 | 0.05 | 1.34 |
| 80 140 | CB30 | Gammex | 0.8 | 0.7 | 1.2 | 3.9 | 0.010 | 0.009 | 0.12 | 3.12 |
| 100 140 | CB30 | Gammex | 0.9 | 0.7 | 1.3 | 4.0 | 0.011 | 0.009 | 0.13 | 3.20 |
| 80 140 | CB50 | Gammex | 0.4 | 0.5 | 0.8 | 3.6 | 0.006 | 0.007 | 0.10 | 3.34 |
| 100 140 | CB50 | Gammex | 0.4 | 0.5 | 1.0 | 3.4 | 0.006 | 0.007 | 0.12 | 3.15 |
| 80 140 | Cortical | Gammex | 1.0 | 0.1 | 2.5 | 0.7 | 0.016 | 0.001 | 0.33 | 0.78 |
| bone | ||||||||||
| 100 140 | Cortical | Gammex | 1.4 | 0.3 | 3.1 | 0.0 | 0.022 | 0.005 | 0.41 | 0.05 |
| bone | ||||||||||
| 80 140 | Tecapeek | Polymer | 1.0 | 0.2 | 0.1 | 13.6 | 0.013 | 0.002 | 0.00 | 10.14 |
| 100 140 | Tecapeek | Polymer | 0.8 | 0.0 | 1.5 | 12.6 | 0.010 | 0.000 | 0.09 | 9.41 |
| 80 140 | Tecaform | Polymer | 1.5 | 0.7 | 0.5 | 10.0 | 0.020 | 0.009 | 0.04 | 7.75 |
| 100 140 | Tecaform | Polymer | 1.3 | 0.6 | 0.2 | 9.4 | 0.018 | 0.008 | 0.01 | 7.31 |
| 80 140 | Tecadur | Polymer | 3.3 | 0.4 | 41.2 | 7.5 | 0.044 | 0.005 | 2.80 | 6.06 |
| 100 140 | Tecadur | Polymer | 3.3 | 0.4 | 41.8 | 7.1 | 0.043 | 0.006 | 2.84 | 5.77 |
| 80 140 | PMMA | Polymer | 0.8 | 0.7 | 0.4 | 10.6 | 0.009 | 0.008 | 0.02 | 7.81 |
| 100 140 | PMMA | Polymer | 0.8 | 0.7 | 1.0 | 10.1 | 0.009 | 0.008 | 0.06 | 7.48 |
| 80 140 | Teflon | Polymer | 3.5 | 0.3 | 1.8 | 16.7 | 0.062 | 0.005 | 0.16 | 16.56 |
| 100 140 | Teflon | Polymer | 3.4 | 0.3 | 1.1 | 16.1 | 0.061 | 0.005 | 0.10 | 15.94 |
| 80 140 | Aluminum | Metal | 1.7 | 2.7 | 4.2 | 33.4 | 0.036 | 0.063 | 0.54 | 55.49 |
| 100 140 | Aluminum | Metal | 1.8 | 2.5 | 4.8 | 32.9 | 0.039 | 0.058 | 0.62 | 54.62 |
| 80 140 | Titanium | Metal | 10.8 | 8.5 | 4.5 | 19.9 | 0.350 | 0.321 | 0.99 | 46.44 |
| 100 140 | Titanium | Metal | 9.4 | 7.0 | 5.0 | 21.4 | 0.307 | 0.264 | 1.11 | 49.75 |
Especially the ϱe/ϱe, w which plays an essential role in radiotherapy can be calculated with an excellent accuracy from DECT images. We do not see a dependency of the ϱe/ϱe, w residuals on the mean atomic number or density as well as we do not see a statistical significant difference between both voltage pairs. Tube currents for both voltage pairs were not similar and therefore it is impossible to evaluate the noise level. It is not the scope of this paper to evaluate noise, filter and voltage pairs and tube currents and we would like to refer the reader to the publications of Saito (2011, 2012). For dose issues from dual source CT compared to single energy CT the reader might be referred to Henzler et al (2012).
3.3. Accuracy of stopping power predictions from DECT images
The SPRw (table 1) could be predicted (equation (3)) for tissue surrogates within a mean accuracy of 0.6% (0.7%) from the measured 80/140Sn kVp (100/140Sn kVp) DECT images (table 3). To predict the SPRw of the lung surrogate only the electron density image was used. This is plausible since the I-value of the lung surrogate is similar to water. For polymers the SPRw was predicted within 2.0% (1.9%) of the 80/140Sn kVp (100/140Sn kVp) images. The SPRw for aluminum was predicted within 1.7% (1.8%), for titanium within 10.8% (9.4%) from the 16-bit reconstructed 80/140Sn kVp (100/140Sn kVp) images. I-values were estimated for the tissue surrogates within a mean accuracy of 3 eV. This might translate into a range uncertainty in the order of ∼0.3% (Andreo 2009, Paganetti 2012).
Figure 2 shows no clear dependence of the SPRw residual to the ϱe/ϱe, w residual. This might be because the residuals of the ϱe/ϱe, w and I-value can compensate each other or add up. Also as mentioned before, elemental compositions are not specifically given for our measured tissue surrogates and there might be an uncertainty in the production process and a subsequent high uncertainty in the reference I-value.
4. Summary and conclusions
DECT imaging enables a purely image based calculation of the electron density relative to water and effective atomic number. With the presented image based algorithm it is possible to calculate both parameters with a very good mean accuracy of 0.4% (ϱe/ϱe, w) and 1.7% (Zeff) in tissue surrogates. Maximum residuals of 0.8% (6.4%) compared to reference values from the manufacturer were detected in the electron density (effective atomic number). With the estimation of I through Zeff, the precise prediction of the SPR for particle therapy using the actual Bethe relation as a basis is feasible. In a first experimental study with tissue surrogates, polymers and metals the DECT based SPR prediction was assessed. This experimental verification in defined materials is a mandatory step to show absolute SPRw errors from DECT prediction before a consideration of patients' DECT data. We believe that DECT has the potential to predict SPRs of materials with unusual elemental compositions like plastic implants or metals. More powerful x-ray detectors with better SNR and modern iterative CT reconstruction algorithms could be of great benefit here. Future comparative dose planning studies with patients' dual and single energy CT images should evaluate its potential in realistic tissue compositions and patients' anatomies.
Acknowledgments
We would like to thank Professor H-P Schlemmer (DKFZ, Division of Radiology) for the continuous support for this project. Martina Jochim (DKFZ, Division of Radiology) is grateful acknowledged for her valuable assistance with the CT image acquisition. NH is funded by the Helmholtz Association.
Footnotes
- 6
In this paper the terms WEPL, SPR and SPRw, are used interchangeably, except in situations where one term is explicitly more correct.



