Abstract
Clinical trials on 177Lu–90Y therapy used empirical activity ratios. Radionuclides (RN) with larger beta maximal range could favourably replace 90Y. Our aim is to provide RN dose-deposition kernels and to compare the tumour control probability (TCP) of RN combinations. Dose kernels were derived by integration of the mono-energetic beta-ray dose distributions (computed using Monte Carlo) weighted by their respective beta spectrum. Nine homogeneous spherical tumours (1–25 mm in diameter) and four spherical tumours including a lattice of cold, but alive, spheres (1, 3, 5, 7 mm in diameter) were modelled. The TCP for 93Y, 90Y and 125Sn in combination with 177Lu in variable proportions (that kept constant the renal cortex biological effective dose) were derived by 3D dose kernel convolution. For a mean tumour-absorbed dose of 180 Gy, 2 mm homogeneous tumours and tumours including 3 mm diameter cold alive spheres were both well controlled (TCP > 0.9) using a 75–25% combination of 177Lu and 90Y activity. However, 125Sn–177Lu achieved a significantly better result by controlling 1 mm-homogeneous tumour simultaneously with tumours including 5 mm diameter cold alive spheres. Clinical trials using RN combinations should use RN proportions tuned to the patient dosimetry. 125Sn production and its coupling to somatostatin analogue appear feasible. Assuming similar pharmacokinetics 125Sn is the best RN for combination with 177Lu in peptide receptor radiotherapy justifying pharmacokinetics studies in rodent of 125Sn-labelled somatostatin analogues.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
General scientific summary Association of a short beta range radionuclide (RN) with a long beta range RN appears well adapted to simultaneously treat small homogeneous and large heterogeneous tumours. Recent clinical trials on 177Lu–90Y therapy used empirical activity ratios and RN with larger beta maximal range could still favourably replace 90Y. Tumour control probabilities (TCP) of several RN in combination with 177Lu were computed for various homogeneous and heterogeneous tumour models. Using 177Lu–90Y with 75–25% activity proportions provided the best TCP. However, 177Lu–125Sn with 80–20% activity proportions achieved significantly better TCP. In addition,125Sn production and its coupling to somatostatin analogue appear feasible. Clinical trials using RN combinations should use RN proportions tuned to the patient dosimetry. 125Sn is potentially the best RN for combination with 177Lu in peptide receptor radiotherapy justifying pharmacokinetics studies in rodents of 125Sn labelled somatostatin analogues.
For more information on this article, see medicalphysicsweb.org
Introduction
Peptide receptor radiotherapy is commonly used in the second or the third line for metastatic neuroendocrine tumours, where each patient usually displays a wide panel of tumour geometries ranging from large heterogeneous tumours down to undetectable millimetric tumours. Patient outcome is intimately linked to the least controlled tumour geometry. It was already shown by O'Donoghue et al (1995) and is commonly known since the introduction of dosimetry software, such as MIRDOSE 3.0 (Stabin 1996), the precursor of OLINDA/EXM (Stabin et al 2005) , that beta emitters with a large range, such as 90Y (12 mm maximal range) are not suitable to cure millimetric tumours. 177Lu, with its shorter beta maximal range (1.5 mm) was already tested by Rames (1959). More recently, in view of its good chelating stability, it was proposed as a better alternative to 90Y for peptide receptor radiotherapy (Kwekkeboom et al 2001) and is now commonly used.
However, when introducing 177Lu, concerns soon appeared about the efficacy of 177Lu to cure heterogeneous tumour where the centre of regions without uptake could not be reached by the beta emitted from the surrounding taking up tissue. Intuitively, it was already admitted that a combination of 90Y–177Lu should be better suited to simultaneously treat small homogeneous tumours and large heterogeneous tumours. The pioneer work of de Jong et al (2005) on a rodent tumour model proved the validity of this concept.
Clinical trials using a 90Y–177Lu combination, both radionuclides (RN) simultaneously given in cocktail or successively in tandem, are now emerging (Frilling et al 2006, Seregni et al 2010, Kunikowska et al 2011, Villard et al 2012). They seem to confirm the previous results obtained in a rodent model. However, the used activity ratios of 177Lu and 90Y, 70–30% (Seregni et al 2010, Villard et al 2012) and 50–50% (Kunikowska et al 2011), were chosen on an empirical basis. Patient outcome should highly profit from an individualized combination of both radiopharmaceuticals (Savolainen et al 2012) . In addition, other RN with larger beta maximal ranges could be superior to 90Y in combination with 177Lu.
The purposes of this study are (i) to provide the dose-deposition kernel of 177Lu (only 177Lu 0.5 mm voxel-based S values have already been reported (Dieudonné et al 2010, Lanconelli et al 2012)) and also of long range beta-emitting RN, i.e. 148Pm, 90Y, 93Y and 125Sn, in order to allow easy voxel-based dose distribution calculation and (ii) to provide the tumour control probability (TCP) using these RN in combination with 177Lu for several tumour models of different diameters including or not, heterogeneities of different sizes, and as recommended by Savolainen et al (2012), while keeping constant the biological effective dose (BED) to the renal cortex that is now widely recognized as the relevant parameter for the kidney toxicity (Barone et al 2005, Wessels et al 2008). A program computing these TCP for personalized parameters choice is also provided in the online supplementary material available at stacks.iop.org/PMB/57/4263/mmedia. These pieces of information should help those who would like to design further trials of combined RN therapy.
Material and method
Dose-deposition kernel
The dose-deposition kernel of 148Pm, 177Lu, 90Y, 93Y and 125Sn point source in water was derived by integration of the mono-energetic beta-ray dose distributions in water computed with Monte Carlo by Cross et al (1992). These integrations were weighted by the respective RN beta spectrum2.
Voxel dose distribution
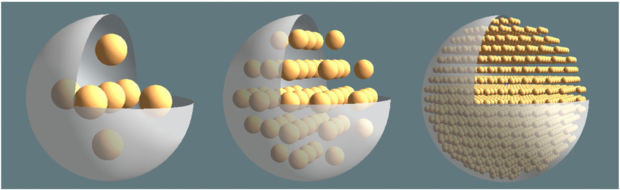
Using a previously validated 3D convolution method (Lhommel et al 2010), the voxel (0.1 mm size on edge)-based dosimetry of the different RN was computed for different tumour models from the dose-deposition kernels derived as described above. Nine homogeneous active spherical tumours with diameters ranging from 1 to 25 mm were modelled. Four heterogeneous 25, 25, 25, 36 mm diameter active spherical tumours were modelled by including identical cold spheres of 1, 3, 5 and 7 mm diameter, respectively, set on the vertices of a cubic grid (figure 1). The distance between the vertices was three times the cold spheres radius so that the edge-to-edge distance between the cold spheres was equal to their radius, i.e. 0.5, 1.5, 2.5 and 3.5 mm, respectively. This ensures that the ratio between the volumes with, and without, RN uptake is almost independent of the heterogeneity size. A minimal distance equal to the cold spheres radius was kept between the edge of the cold sphere and the edge of the housing sphere. The housing sphere diameter was increased to 36 mm for the 7 mm heterogeneity in order to allow setting of more than one cold sphere.
Figure 1. Open view in 3D rendering of the 25 mm diameter heterogeneous tumour including 5, 3 and 1 mm diameter (from left to right) cold spheres located on a cubic grid.
Download figure:
Standard imageVoxel dose distribution of 90Y, 93Y, 148Pm and 125Sn in combination with 177Lu
Three assumptions were made in all simulations: (i) tissue uptakes and pharmacokinetics expressed as a percentage of the activity injected to the patient are independent of the RN and of the labelled peptide, (ii) the specific activity in tumour voxels taking up the labelled compound is constant in the tumour and is independent of the tumour diameter and of the heterogeneity pattern, (iii) all the RN pharmacokinetics follow a mono-exponential time course with a biological half-life equal to the mean biological half-life of 86Y-DOTATOC that was observed using amino acid infusion in a previously published phase I study in patients with neuroendocrine tumours (Jamar et al 2003), i.e. 210 and 92 h for the tumours and kidneys, respectively.
Different RN combinations were generated while keeping constant the renal cortex BED to 11 Gy per cycle that is one-third of the maximal renal cortex safe dose, which should be reached for the whole therapy (Wessels et al 2008). According to the activity measured ex vivo on a human kidney (Konijnenberg et al 2007), 70% and 30% of the total kidney activity were assumed to be in the cortex and in the medulla, respectively. Using these assumptions, the renal cortex dosimetry was assessed using the MIRD pamphlet no 19 kidney model (Bouchet et al 2003) included in OLINDA/EXM (Stabin et al 2005) . Table 1 shows, for the different RN, the effective half-lives in the tumour (Ttume1/2) and in the kidney (Tkide1/2) and the renal cortex dose  , i.e. the absorbed dose per 1 MBq of the total activity in the kidney. The BED is
, i.e. the absorbed dose per 1 MBq of the total activity in the kidney. The BED is

where the Lea–Catcheside (McParland 2010) factor G is
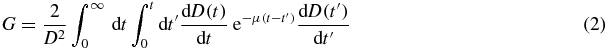
with α/β = 2.6 Gy for the quadratic model parameter and µ = ln(2)/2.8 h for the DNA enzyme repair rate of the glomerular cells (Barone et al 2005, Wessels et al 2008). Reading equation (2) from right to left, the absorbed dose rates  and
and  describe the probabilities that two independent particle tracks consecutively induce a DNA single-strand break at the times t' and t (t'<t). The exponential term
describe the probabilities that two independent particle tracks consecutively induce a DNA single-strand break at the times t' and t (t'<t). The exponential term  sandwiched in-between accounts for the probability that the enzyme system can repair the first DNA single-strand break before the second occurs, hence reducing the occurrence of a lethal DNA double-strand break. The double-integration handles that this scenario can occur at any time t' and t (t'< t). The absorbed dose rate for a combination with kidney activities AR and AL at t = 0 for the RN R and L is
sandwiched in-between accounts for the probability that the enzyme system can repair the first DNA single-strand break before the second occurs, hence reducing the occurrence of a lethal DNA double-strand break. The double-integration handles that this scenario can occur at any time t' and t (t'< t). The absorbed dose rate for a combination with kidney activities AR and AL at t = 0 for the RN R and L is
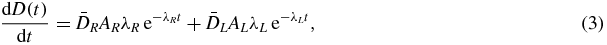
where λ = ln (2)/Tkide1/2 for each RN. The calculation of the BED using equations (1)–(3) gives a quadratic relation between the activities AR and AL in order to keep the BED constant. Using the previous assumptions, two expressions atumR(x) and atumL(x) can be found for the tumour-specific activities of the RN R–L combination x (0 ≤ x ≤ 1) keeping the BED constant (see the appendix).
Table 1. Tumour and kidney effective half-lives (in bold), and renal cortex dose  per MBq of total kidney activity (see 'Material and method').
per MBq of total kidney activity (see 'Material and method').
| T1/2 decay (h)↓ | Tumour Ttume1/2(h) | Kidney Tkide1/2(h) | Tumour/Kidney ratio | 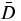 (mGy/MBq) (mGy/MBq) |
|
|---|---|---|---|---|---|
| T1/2 biol (h) → | 210.0 | 92.0 | 2.28 | ||
| 93Y | 10.2 | 9.7 | 9.2 | 1.06 | 27 |
| 90Y | 64.2 | 49.1 | 37.7 | 1.30 | 89 |
| 148Pm | 129.6 | 80.1 | 53.8 | 1.49 | 107 |
| 125Sn | 232.2 | 110.3 | 65.9 | 1.67 | 139 |
| 177Lu | 161.8 | 91.1 | 58.5 | 1.56 | 24 |
Tumour control probability
The TCP was computed using the formalism developed by Niemierko (1999) that includes two steps. The computation of the equivalent uniform dose (EUD):

where vi is the fraction of living cells that received an absorbed dose Di. Afterward the TCP is computed by

TCD50 is the tumour dose to control 50%, i.e. TCP = 0.5, of the tumours when they are homogeneously irradiated, γ50 and a are unitless model parameters that are tumour specific. We used the values 60 Gy, 2 and −12 experimentally found by Gay and Niemerko (2007) for these parameters, respectively. In the computation of TCP using equations (4) and (5), the density of living cells was assumed to be constant in the tumour, even in the regions without uptake. However, the program provided in the online supplementary material available at stacks.iop.org/PMB/57/4263/mmedia allows changing this density, the Niemerko's parameters, and the kidney and tumour biological half-lives.
Disease control probability
Assuming that the disease of the patient is controlled only if his tumours are all controlled, we obtain

where TCPi is the TCP of the tumour i and the product is performed on all the tumours of the patient.
Results
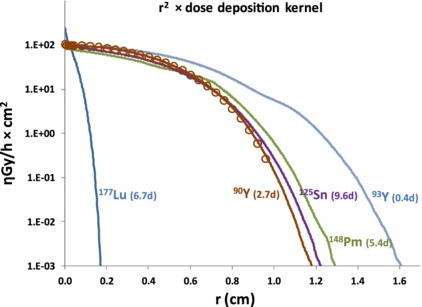
Figure 2 shows the dose-deposition kernels times the square of the distance r (this common representation well represents that at a distance r, a point is irradiated by a surrounding shell of area 4πr2). See the online supplementary material available at stacks.iop.org/PMB/57/4263/mmedia for numerical values of the 177Lu, 93Y, 148Pm and 125Sn dose kernels. For comparison, the 90Y dose kernel computed by Cross et al (1992) using Monte Carlo was also shown (open circles).
Figure 2. Dose-deposition kernel times the square of the distance r from a 1 Bq point source in water of 177Lu, 125Sn, 90Y, 93Y, 148Pm here derived by computation (lines) and of 90Y extracted from Cross et al (1992) (open circle). Decay half-lives are noted in brackets.
Download figure:
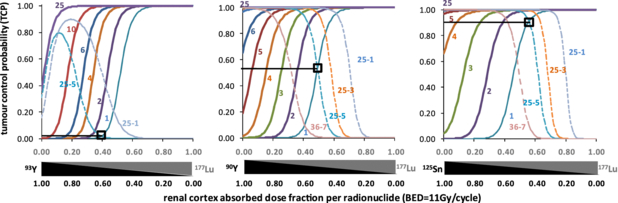
Standard imageFigure 3 shows the TCP as a function of the different RN combinations for a therapy course giving a constant renal cortex BED of 11 Gy per cycle. The tumour-initial-specific activities couple (atumL(0), 0), i.e. 100% of 177Lu, corresponding to a mean absorbed dose of 180 Gy to the homogeneous tumour of 25 mm in diameter for the whole therapy, i.e. ideally three cycles. Note that using 90Y–177Lu, homogeneous 2 mm tumour and 25 mm tumours with 3 mm heterogeneity can simultaneously be controlled (TCP > 0.9) using the combination providing a renal cortex absorbed dose with 50% originating from 177Lu and 50% from 90Y (as being quadratic, BED cannot be decomposed into two components). 125Sn–177Lu achieved a better result by simultaneously controlling homogeneous 1 mm tumour and tumours with 5 mm heterogeneity. 93Y–177Lu displayed the lowest TCP. The TCP obtained for this tumour-heterogeneity size couple were 0.02, 0.55 and 0.93 for 93Y–177Lu, 90Y–177Lu and 125Sn–177Lu respectively. 148Pm provided results that were better than 90Y but inferior to 125Sn (data not shown).
Figure 3. TCP for different RN combinations providing a mean absorbed dose of 180 Gy to the 25 mm-diameter homogeneous tumour with pure 177Lu. The horizontal axis gives the proportions of the renal cortex absorbed dose originating from each RN, while keeping the BED to 11 Gy per cycle. The TCP of homogeneous tumours are the continuous lines labelled with the tumour diameter in mm and the TCP of heterogeneous tumours are the dashed lines labelled with the tumour diameter and the size of the heterogeneities, in mm. The open squares show the TCP that can simultaneously be obtained for the couple (1 mm tumour, 5 mm heterogeneity).
Download figure:
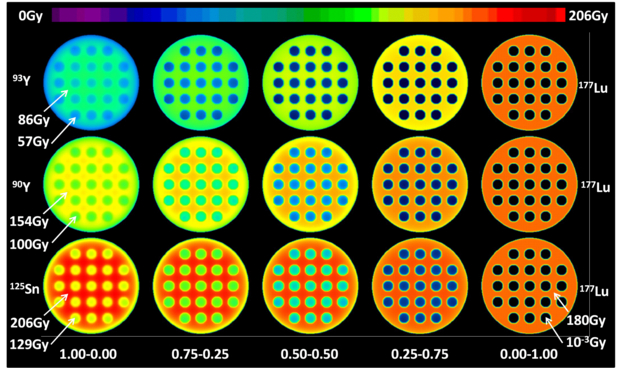
Standard imageFigure 4 shows the central slice of the voxel dose distribution for the 25 mm diameter sphere including 3 mm diameter cold spheres heterogeneity for the previous 180 Gy mean absorbed dose simulations. Pure 125Sn provided the best irradiation of the inserted cold spheres and of the region between the cold spheres as well, while pure 177Lu provided the best irradiation of the inner edge of the 25 mm diameter sphere. Note the increasing absorbed dose in the region between the cold spheres when 177Lu is progressively replaced by 125Sn, while keeping the renal cortex BED constant as a result of the longer 125Sn decay half-life combined with the tumour washout that is slower to that of the kidney.
Figure 4. Central slice of the voxel dose distribution for different RN combinations shown in figure 3 for the 25 mm diameter sphere with 3 mm diameter heterogeneities. All combinations provide the same renal cortex BED. Couple numbers give the fraction of the absorbed dose to the renal cortex originating from either RN.
Download figure:
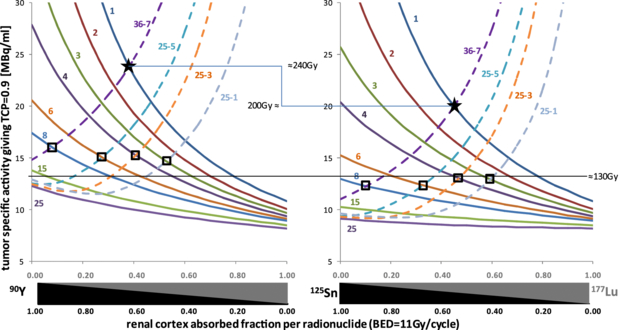
Standard imageFigure 5 shows the tumour-specific activity (in the area that takes up the RN) required when 177Lu is injected alone in order to ensure a TCP of 90% for the different tumour models and for a therapy giving a constant renal cortex BED of 11 Gy per cycle. Regarding the tumour washout half-life and the 177Lu beta energy, 15 MBq ml−1 conveniently corresponds to about 150 Gy for 177Lu alone (Stabin et al 2005). For a same tissue uptake, 125Sn–177Lu ensures a better simultaneous control of homogeneous and heterogeneous tumours. Using the appropriate RN proportions, 90Y–177Lu requires an additional uptake corresponding to 40 Gy than that required by 125Sn–177Lu to both controls 1 mm tumour and 7 mm heterogeneities. A program computing these curves for personalized choice of the Niemerko parameters, of the biological half-lives, of the threshold TCP, of the cell living density and of the therapy cycle BED is provided in the online supplementary material available at stacks.iop.org/PMB/57/4263/mmedia. When giving the therapy in one cycle with the maximal safe renal cortex BED, i.e. 33 Gy, the efficiency still improves a little bit in favour of 125Sn–177Lu (see the figure in the online supplementary material available at stacks.iop.org/PMB/57/4263/mmedia).
Figure 5. Required specific activity in the tumour at the time of the maximum RN uptake and when 177Lu is injected alone, in order to achieve a TCP of 90% for the different combinations giving a renal cortex BED of 11 Gy per cycle. The tumour-specific activity changes when 177Lu is progressively replaced by the other RN as the renal cortex BED is kept constant. The TCP of homogeneous tumours are the continuous lines labelled with the tumour diameter and the TCP of heterogeneous tumours are the dashed lines labelled with the tumour diameter and the size of the heterogeneities (all in mm). The stars represent the combinations and minimal specific activities required to concomitantly control 1 mm tumours and 7 mm heterogeneity. The open squares represent the different homogeneous tumour diameters and heterogeneity sizes couples that can be controlled using 125Sn–177Lu, but not using 90Y–177Lu, for a specific activity of 12.5 MBq ml−1.
Download figure:
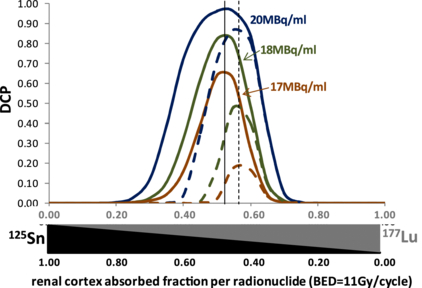
Standard imageFigure 6 shows the disease control probability (DCP) for different tumour-specific activities in a function of the 177Lu–125Sn combination proportions in two patients owning one 25 mm diameter tumour including 5 mm diameter heterogeneities jointly with one and ten 1 mm diameter homogeneous tumours, respectively. Due to the fast decrease of the TCP in heterogeneous tumours (see figure 3), only a small shift of the optimal combination proportions in favour of 177Lu is observed for the patient burdened with ten 1 mm tumours. However, for this patient the maximal DCP decreases faster with the tumour-specific activity. The program provided allows computing DCP for personalized number of tumours.
Figure 6. DCP for different tumour-specific activities when 177Lu is injected alone in function of the 177Lu–125Sn proportion giving a renal cortex BED of 11Gy per cycle in two patients owning one 25 mm diameter tumour including 5 mm diameter heterogeneities jointly with one (continuous lines) and ten (dashed lines) 1 mm-diameter homogeneous tumours, respectively.
Download figure:
Standard imageDiscussion
The simple, but challenging, tumour model studied here clearly shows the benefit of using 90Y–177Lu combination rather than a single RN alone. This is in line with the experimental observations of de Jong et al (2005). The combination providing a 50–50 renal cortex absorbed dose originating from 177Lu and 90Y appears to be a good compromise: for a mean tumour delivered dose of 180 Gy, this combination simultaneously allows controlling small homogeneous tumours down to 2 mm diameter, as well as heterogeneities up to 3 mm diameter. This combination expressed as a fraction of RN activities to inject corresponds to a 75% 177Lu–25% 90Y cocktail. This is close to the combination used by Seregni et al (2010) , but is quite different from the 50–50% tandem used by Kunikowska et al (2011). For a tumour mean delivered dose of 180 Gy, this last proportion controls 7 mm heterogeneities, but not tumours smaller than 5 mm in diameter.
For the time being, 90Y is not yet proposed in combination with 131I internal radiotherapy. 131I owning a beta range and a half-life almost similar to those of 177Lu, the previous considerations are expected to be valid for 90Y combination therapy with 131I. Bone marrow toxicity was not addressed here; however, knowing the bone marrow pharmacokinetics for the different RN, the present methodology could be applied by studying the combination proportions that keep the most critical tissue, i.e. renal cortex or bone marrow, to its maximal safe BED. Note that this most critical tissue could change when moving from pure 177Lu to pure 90Y (or 125Sn) proportion.
The results also show that there is no significant difference between a tumour mean absorbed dose of 130 and 200 Gy when a single RN is used: for both absorbed doses, 90Y cannot control tumours smaller than 6 mm, and for both absorbed doses, 177Lu controls all homogeneous tumours, but not heterogeneity (figure 5(left)). On the other hand, using a RN combination, the situation dramatically changes between these two absorbed doses: 130 Gy can only control 6 mm tumours and 1 mm heterogeneity together, while 200 Gy can simultaneously control 1 mm tumour and 5 mm heterogeneity. As the 86Y-SMT-487 trial showed huge differences in renal uptake (Barone et al 2005, Jamar et al 2003), it is of paramount importance to individually assess this uptake to inject the most efficient safe dose to each patient. Of course, this uptake should be evaluated separately for each RN. 177Lu is easily imaged by SPECT, and the 90Y uptake can be accurately assessed after the first therapy cycle either by PET (Walrand et al 2010) or by bremsstrahlung pinhole SPECT (Walrand et al 2011). In addition, curves of figure 5 can be individually computed from patient-specific tumour and kidney dosimetry using the program provided in the online supplementary material available at stacks.iop.org/PMB/57/4263/mmedia. This may allow assessing the individual optimal RN proportions.
Despite its large beta range (17 mm), 93Y performed less well than 90Y. This is linked to its short half-life (10 h) that does not enable to benefit from the differential washout between the kidney and the tumours (table 1). The same behaviour was noted for 66Ga (30 mm, 9.5 h) and 72Ga (19 mm, 14 h) (data not shown). Note that in the case of 93Y, the comparison is entirely valid due to its identical chemical properties to those of 90Y or 86Y. The comparison will have to be refined for 125Sn when biodistribution and pharmacokinetics can be assessed.
After making these observations, we searched using the LNBL isotopes tool (see footnote 2) and analyse the beta spectrum of RN with a half-life longer than that of 90Y and with a beta end point energy higher than 1.7 MeV. Nine RN came out from this search (table 2), including only two RN, 148Pm and 125Sn, still having a significant beta abundance of end point energy up to 2 MeV. However, 125Sn had a significantly longer half-life and performed not only better than 90Y, but also better than 148Pm (data not shown). As expected 125Sn provided the best tumour control: for 180 Gy, 1 mm tumour and 5 mm heterogeneities can be controlled (figure 3). Furthermore, for 200 Gy 1 mm tumour and 7 mm heterogeneities can be simultaneously controlled (figure 5(right)).
Table 2. Beta emitter with a half-life (HL) longer than that of 90Y and with a beta energy end point (Ep) higher than 1.7 MeV: ME—mean energy; EM—energy of the maximum abundance; E1/10—energy with abundance corresponding to 1/10 of the maximal abundance. Numbers in grey background are exclusion criteria. Numbers in bold show the best values between the two acceptable 90Y surrogates.
| HL (d) | EP (MeV) | ME (MeV) | EM (MeV) | E1/10 (MeV) | |
|---|---|---|---|---|---|
| 90Y | 2.7 | 2.3 | 0.9 | 0.8 | 2.1 |
| 47Ca | 4.5 | 2.0 | 0.4 | 0.2 | 0.7 |
| 148Pm | 5.4 | 2.5 | 0.7 | 0.3 | 2.1 |
| 125Sn | 9.6 | 2.4 | 0.8 | 0.0 | 2.0 |
| 126Sb | 12.5 | 1.9 | 0.3 | 0.0 | 0.8 |
| 32P | 14.3 | 1.7 | 0.7 | 0.6 | 1.5 |
| 156Eu | 15.2 | 2.5 | 0.4 | 0.0 | 0.4 |
| 86Rb | 18.7 | 1.8 | 0.7 | 0.3 | 1.6 |
| 124Sb | 60.2 | 2.3 | 0.4 | 0.0 | 0.6 |
| 160Tb | 72.3 | 1.7 | 0.2 | 0.0 | 0.6 |
125Sn has the drawback to decay into a beta emitter: 125Sb (2.7 years) that afterwards decays to the stable isotope 125Te. The total 125Sb activity produced will be 1% of the 125Sn activity (half-life ratio 232 h/2.7 years). Due to the long 125Sn half-life (232 h) compared to the kidney washout half-life (92 h), only a small part of the 125Sn will decay into 125Sb in the kidney. For the time being, the exact computation of the additional irradiation induced by the progeny 125Sb is not possible owing to the fact that pharmacokinetics of 125Sn-labelled peptides were not assessed yet. However, even if all the 125Sb produced in the kidney is retained, a simple computation shows that 125Sb will only give a maximal additional kidney absorbed dose of 10% than that given by 125Sn. 117mSn-DTPA has been used in bone pain palliation (Bishayee et al 2000) and has shown a good stability in vitro and in vivo (Srivastava et al 1985). Peptide labelling with 125Sn is thus likely feasible.
125Sn is a fission product, but can also be produced by fast or thermal neutrons irradiation of the stable isotope 124Sn in the same way as 177Lu. 125Sn for the time being has no application and was produced only in small amounts for the fundamental nuclear physics research. Natural tin is a mix of the stable isotopes 112,114–120,122,124Sn. However, irradiation of 124Sn-enriched targets (Krane and Sylvester 2006, Mayer et al 1992) by thermal neutron, followed by 10 days cooling, produces 125Sn almost free of other radioisotopes (<1%). However, cross sections appear limited for a medical production. Table 3 shows the typical 125Sn activity produced in the 99Mo/99mTc generator manufacturing by thermal neutron irradiation of 235U (Mushtaq et al 2009). Tin RN can be rapidly extracted and purified from the fission products (Erdal and Wahl 1968). The short half-lived 121,127,128Sn can be removed by physical decay, the other beta emitter contaminants to be taken into account in the therapy dosimetry planning.
Table 3. Tin RN activity in waste 10 days after 12 h thermal neutron irradiation of 235U to produce 4000 GBq of 99Mo, i.e. 200 × 20 GBq 99Mo/99mTc generators (extracted from Mushtaq et al (2009))). IT: isomeric transition.
| MBq | Decay mode | Progeny | |
|---|---|---|---|
| 119mSn (293 d) | 1 | IT (γ 25 keV, 30%) | Stable |
| 121Sn (27 h) | 40 | β (0.4 MeV, 22%) | Stable |
| 123Sn (129 d) | 64 | β (1.4 Mev, 100%) | Stable |
| 125Sn (10 d) | 1320 | β (2.4 Mev, 100%) | 125Sb (2.7 years) |
The present simple tumour model showed the benefit of 125Sn–177Lu versus 90Y–177Lu. However, the determination of the optimal combination proportion for clinical therapy requires more realistic tumour modelling. Unfortunately, heterogeneity of neuroendocrine tumours in humans is different from preclinical therapy model (Marcos et al 2002) and up to now structural and RN imaging at cellular level can be obtained only by ex vivo techniques. Tumour RN distribution depends not only on the receptor density distribution as shown by autoradiography after ex vivo incubation with the labelled peptide but also depends on the vascular tumour system. Direct data from patients injected with 111In-octreotide would be extremely useful to further explore this avenue and obtain realistic tumour heterogeneity models. Finally, proposed refinement of TCP calculation (Hobbs et al 2011) to account for statistical fluctuations associated with low activities should be considered.
Conclusion
This study strongly supports the merit to perform clinical trial using the 90Y–177Lu combination with a RN proportion optimized from a patient-specific tumour and kidney dosimetry. The latter point is of prime importance in order to inject the most efficient safe activity with the optimal RN proportion in view of the fact that a small reduction of the tumour absorbed dose can drastically knock down the tumour control. This study confirms that 90Y is a good RN that can easily be used in combination with 177Lu.
According to published data, production of 125Sn and its coupling to somatostatin analogue appear feasible. From a physical viewpoint, 125Sn is the best RN for internal radiotherapy in combination with 177Lu and provides significantly better tumour control than using 90Y. This result is based on the assumption that the pharmacokinetics of peptides labelled with 90Y and 125Sn are similar. It is thus of prime importance to challenge this assumption. The first step should be to assess the biodistribution and pharmacokinetics of 125Sn-labelled somatostatin analogues in rodents. The same concept can be applied to other human tumour models known to contain large heterogeneities. Finally, the biological behaviour of its progeny 125Sb has also to be assessed in order to refine the simulation of the radioprotection issues.
: Appendix
The absorbed dose is
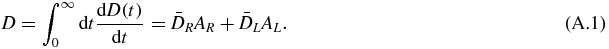
The double integration in equation (2) gives
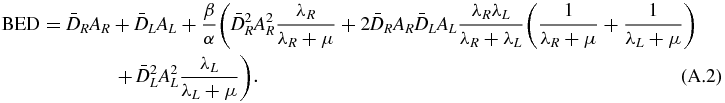
Equation (A.2) can be rewritten as

with

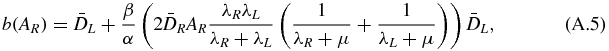
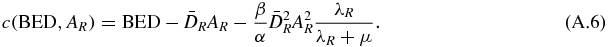
As a, b and c are positive, the solution of equation (A.3) is
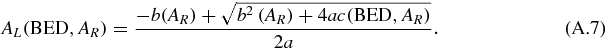
Let start with a combination composed by 100% of the RN L = 177Lu, i.e. AR = 0. The 177Lu activity Al that gives a kidney BED to the cortex is AL(BED, 0). Then, equation (A.7) gives the activity of 177Lu in the combination in order to keep BED constant when the activity of the RN R is progressively increased up to AmaxR, i.e. AL(BED, AmaxR) = 0.
Let assume that for this combination composed by 100% of the RN L = 177Lu, the tumour-initial-specific activity is atumL. Then, resulting from assumption (i) the tumour-specific activity couple (atumL, aRtum) at t = 0 of the RN combination x giving the kidney activity couple (AL(BED, xAmaxR), xAmaxR) at t = 0 to the kidney with 0 ≤ x ≤ 1 is given by
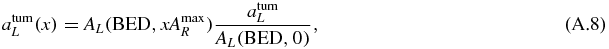

Note that in the combination made of 100% of the RN R, atumR(1) can be greater or smaller than atumL depending on the decay properties of the RN R.