Abstract
The surface morphology, structure and composition of human dentin treated with a femtosecond infrared laser (pulse duration 500 fs, wavelength 1030 nm, fluences ranging from 1 to 3 J cm−2) was studied by scanning electron microscopy, x-ray diffraction, x-ray photoelectron spectroscopy and Fourier transform infrared spectroscopy. The average dentin ablation threshold under these conditions was 0.6 ± 0.2 J cm−2 and the ablation rate achieved in the range 1 to 2 µm/pulse for an average fluence of 3 J cm−2. The ablation surfaces present an irregular and rugged appearance, with no significant traces of melting, deformation, cracking or carbonization. The smear layer was entirely removed by the laser treatment. For fluences only slightly higher than the ablation threshold the morphology of the laser-treated surfaces was very similar to the dentin fracture surfaces and the dentinal tubules remained open. For higher fluences, the surface was more porous and the dentin structure was partially concealed by ablation debris and a few resolidified droplets. Independently on the laser processing parameters and laser processing method used no sub-superficial cracking was observed. The dentin constitution and chemical composition was not significantly modified by the laser treatment in the processing parameter range used. In particular, the organic matter is not preferentially removed from the surface and no traces of high temperature phosphates, such as the β-tricalcium phosphate, were observed. The achieved results are compatible with an electrostatic ablation mechanism. In conclusion, the high beam quality and short pulse duration of the ultrafast laser used should allow the accurate preparation of cavities, with negligible damage of the underlying material.
Export citation and abstract BibTeX RIS
1. Introduction
Minimally invasive dentistry is a new approach to dental care that integrates prevention, early caries diagnosis and remineralization of carious lesions, as well as minimization of the amount of dentin removed in case of cavitation. Lasers are important at all stages of this treatment methodology because their bactericidal action allows eliminating cariogenic bacteria and, if cavitation has occurred and no remineralization is possible, removing the carious tissue accurately, with negligible degradation of the remaining tissue [1, 2]. The initial research efforts in laser dentistry were based on continuous wave or pulsed CO2 [3–7] and Nd : YAG lasers [4, 8–10], with radiation wavelengths of 10.6 µm and 1.064 µm, respectively. Continuous wave lasers do not allow accurately controlling the radiation-tissue interaction time at the very short time scale required for effective ablation of dental tissues and lead, in general, to unacceptable warming up of the tooth, potentially leading to necrosis, and extensive carbonization and melting of the laser-treated region [6, 11]. In the case of pulsed lasers the interaction time is controlled by the pulse duration. Pulsed CO2 and Nd : YAG lasers allow for pulse durations in the 10−3–10−8 s range, which are sufficiently long for significant heat conduction to the bulk to occur, still leading to thermal effects such as surface melting, thermal degradation of dental tissues, carbonization and thermomechanical cracking [12, 13]. To minimize thermal effects, excimer and erbium lasers, which operate in the ultraviolet and infrared range, respectively, have also been investigated. Vilar and co-workers [14–16] studied the ablation and surface texturization of dentin and enamel by KrF excimer laser radiation (λ = 248 nm) and concluded that, in contrast with infrared lasers, the mechanism of intertubular dentin removal is predominantly photochemical (disruption of collagen molecules due to covalent bond breaking by the high energy UV photons), thus substantially reducing thermal damage. However, due to their high cost, the potentially harmful effect of intense ultraviolet radiation [17], and low ablation rate [14] UV lasers do not comply with dental application requirements and erbium lasers are usually preferred. In this moment, Er : YAG, Er : YSGG and Er,Cr : YSGG lasers operating at 2.94, 2.79 and 2.78 µm, respectively, are widely used for the treatment of caries. Since there is a strong peak in the absorption spectrum of water and OH− at around 3 µm, the radiation of these lasers is efficiently absorbed by dental tissues, in particular by intertubular dentin, which contains a large proportion of water in its constitution [18]. As a result, water in the dentinal structure is suddenly warmed up and evaporates, originating a sudden pressure wave that mechanically disrupts the dental tissues, causing their removal. Employing this type of lasers effectively reduces purely thermal effects and early results [19] indicate that, within certain ranges of the laser parameters, carious dentin can be removed without damaging the underlying tissue and the dental structure. However, more recently, it has been demonstrated that, under certain conditions, the mechanical shock arising from the explosive evaporation of water lead to sub-superficial cracking of dentin and a decrease of the restoration/tooth adhesion [20, 21]. Moreover, these sub-superficial microcracks allow the infiltration of dentin by salivary fluids and its colonization by cariogenic bacteria, thus increasing the risk of secondary caries [20, 22].
In contrast with micro- and nanosecond pulse duration lasers, the pulse duration of femtosecond lasers is shorter than characteristic energy relaxation times such as the electron-lattice energy transfer time and the heat conduction time. As a result, no atomic or ionic motion can occur during such a short laser pulse and conventional thermal and thermomechanical ablation processes are minimized [23]. Moreover, if the laser intensity exceeds 1013–1014 W cm−2, ablation proceeds via an electrostatic mechanism [23], entirely avoiding thermal effects in the remaining dental tissues and warming up of the pulp, as well as suppressing thermomechanical cracking. The wider availability and decreasing cost of ultrafast lasers in recent years opens new perspectives for minimally invasive laser dentistry.
Laser ablation of enamel and dentin by femtosecond lasers was previously studied by several authors. Neev et al [24] compared the ablation rate in the femtosecond and the nanosecond regimes using a Ti : sapphire chirped pulse amplifier (CPA) laser system with 1.05 µm radiation wavelength capable of providing laser pulses of 350 fs and 1 ns and concluded that the ablation rate for 350 fs pulse duration is much higher than for 1 ns laser pulses (0.8 µm mJ−1 at 1.0 J cm−2 as compared with 0.11 µm mJ−1 at 34 J cm−2), thus allowing much faster dental treatment. On the other hand, collateral damage was minimized and the tubules in the ablated surface created with femtosecond pulses were fully exposed and only about 10% of the crater showed evidence of melting. The ablation of dental enamel in the femtosecond pulse duration regime was studied by Rode et al [25] using a Ti : sapphire laser with 800 nm radiation wavelength, 95–150 fs pulse duration and 1 kHz repetition rate. They concluded that the ablation rate increases linearly with the radiation fluence above an ablation threshold of 2.1 ± 0.1 J cm−2. The in vitro intrapulpal temperature rise during the preparation of a cavity for a total duration of 200 s was about 10 °C, but this value could be decreased below the pulpal damage threshold of 5.5 °C by cooling the tooth with forced air flow.
The results achieved up to now show that femtosecond lasers have considerable potential for dentistry applications, because their short pulse duration (∼10−13 s) allows achieving very high dentin removal rates while minimizing thermal and thermomechanical effects. Nevertheless, despite the research already carried out, no studies on the influence of femtosecond laser processing on the microstructure and constitution of dentin exist up to this moment. This is the object of this work. The results obtained show that the ablation surfaces present an irregular and rugged appearance, with no significant traces of melting, deformation, cracking or carbonization, and that the dentin constitution and chemical composition is not significantly modified by the laser treatment. These results are compatible with the theoretical predictions based on the electrostatic ablation model [26], suggesting that ablation is electrostatic, hence essentially athermal.
2. Materials and methods
Sample preparation. Approximately 2 mm thick dentin discs were cut from human molar teeth, parallel to the occlusal plane, using a low speed diamond saw. One of the discs' faces was polished with 600 mesh SiC paper under flowing water lubrication. After preparation, the samples were stored in a 5% chloramine solution until laser processing was carried out.
Laser treatment. The laser treatment was performed in air, using an Yb : KYW chirped-pulse-regenerative amplification laser system, with pulse duration of about 500 fs, a radiation wavelength of 1030 nm and a pulse-to-pulse instability of less than 1%. The experiments were carried out by translating the sample relatively to the stationary laser beam using a computer controlled XY stage or with a stationary sample. The laser beam was kept perpendicular to the sample surface and focused with a 100 mm focal length lens. In order to treat large areas, the sample was moved along linear tracks with a scanning velocity of 5 mm s−1. Area coverage was achieved by lateral displacement of the table by about 10% of the track width at each step. As a result, each irradiated zone was exposed to about 200 pulses. The laser treatment was carried out with average fluences of 1, 1.8 and 3 J cm−2. All experiments (stationary and non-stationary) were performed using a pulse repetition rate of 1 kHz.
Scanning electron microscopy analysis. The surface topography was investigated by scanning electron microscopy (SEM) in the secondary electrons imaging mode, using a FEG-SEM JEOL 7001 field-emission gun scanning electron microscope operated at an electron acceleration voltage of 10.0 kV. To avoid charging effects, the SEM samples were coated with gold by dc sputtering prior to observation. The ablation fluences were calculated from the depth and dimensions of the ablation craters obtained in the stationary mode. In turn, these parameters were determined by 3D reconstruction based on stereo pairs of SEM images (tilting angles of 0° and 10°) using Alicona-Mex® software.
X-ray photoelectron spectroscopy. The x-ray photoelectron spectroscopy (XPS) analysis was performed using an ESCALAB 200A VG Scientific (UK) spectrometer with the PISCES software for data acquisition and analysis. The experiments were carried out using an achromatic Al (Kα) x-ray source operated at 15 kV (300 W), at a pressure lower than 10−6 Pa. The spectrometer was calibrated with reference to Ag 3d5/2 (368.27 eV) and operated in the constant analysis energy mode with 20 eV pass energy. The spectra were analysed by fitting the experimental data with Gaussian–Lorentzian shaped peaks and using a Shirley-type background correction.
Fourier transform infrared spectroscopy. The Fourier transform infrared (FTIR) spectroscopy analysis was performed in transmittance. Small samples of dentin were carefully scraped from the surface of the specimen before and after the laser treatment using a scalpel and conditioned in a diamond cell. The infrared spectra were recorded in the 4000–650 cm−1 range, with a 4 cm−1 resolution, by accumulating 256 wavelength scans.
X-ray diffraction. The mineralogical constitution of dentin samples surfaces before and after laser treatment were analysed by x-ray diffraction (XRD) using a Siemens D5000 diffractometer in glancing incidence (1°). The scanning range was 20° to 80° and the scanning speed 2.4° min−1.
3. Results
3.1. Ablation threshold and ablation rate
The ablation threshold for dentin was determined by submitting a number of samples to a single laser pulse with increasing fluence and observing the laser-treated surface by SEM. It was estimated to be 0.6 ± 0.2 J cm−2. The ablation rate was calculated from measurements of the depth of ablation craters, for different radiation fluences and number of pulses, for stationary and moving samples, under dry conditions or with water refrigeration. The exact ablation rate depended on the surface treatment strategy (stationary or moving sample) but was typically in the range 1–2 µm/pulse for an average fluence of about 3 J cm−2. Figure 1 depicts a SEM micrograph of a typical ablation spot obtained in stationary mode and its corresponding 2D topographic profile generated with Alicona-Mex® software.
Figure 1. Cavity created with 35 laser pulses and with average fluence of 3 J cm−2 in stationary mode: (a) SEM image of top view and (b) typical 2D topographical profile.
Download figure:
Standard image3.2. Surface morphology
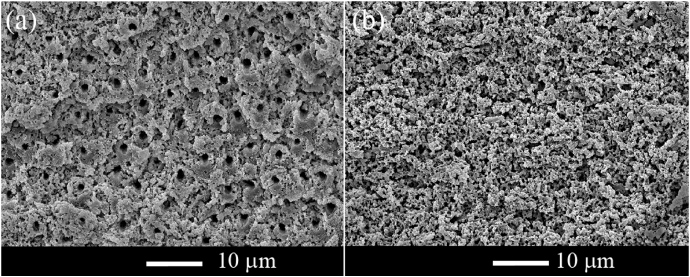
The SEM micrographs depicted in figures 2 and 3 show the morphology of large area dentin surfaces treated with average pulse fluences of 1 J cm−2 and 1.8 J cm−2, respectively (larger than the ablation threshold). Independently of the fluence used, the treated surfaces are flat and, at close view, present an irregular and rugged, appearance. The smear layer was entirely removed by the laser treatment and the dentinal tubules are exposed and open. For a fluence of 1 J cm−2 an extremely flat and smooth surface results from the laser treatment (figure 2(a)). Observation with higher magnification (figure 2(b)) shows that the morphology of the dentinal structure is clearly preserved. No traces of melted material are observed in the SEM image. The peritubular dentin sheets are clearly visible, revealed as flat cross-sectional surfaces, as if they had undergone brittle fracture perpendicularly to the tubule axis. The areas corresponding to intertubular dentin present a scaly and relative porous surface. In some areas, the intertubular dentin sheets protrude above the surface but no traces of deformation are observed anywhere at the surface. A few loose particles with an angular irregular shape are observed on the surface, suggesting that fractured debris is ejected from the surface as a result of the laser pulse and fall back on the surface. Microcracks are observed in some of the peritubular dentin sheets (figure 2(c)).
Figure 2. SEM micrographs of a large area dentin surface after a laser treatment with an average fluence of 1 J cm−2: (a) low magnification image; (b) and (c) high magnification images showing the overall morphology of the surface. Redeposited particles and cracks in the peritubular dentin sheets are indicated by white arrows in (c).
Download figure:
Standard imageFigure 3. SEM micrographs of a large area dentin surface after a laser treatment with an average fluence of 1.8 J cm−2: (a) at the centre; and (b) at the periphery of the treated area.
Download figure:
Standard imageSEM images of dentin surfaces treated with 1.8 J cm−2 are shown in figure 3. The images in this figure, with similar magnification, were taken at the centre (figure 3(a)) and periphery (figure 3(b)) of the laser-ablated region. In the region treated with maximum irradiance (near the centre of the beam) the surface morphology is similar to the one observed in figure 2, for samples treated with average fluence of 1 J cm−2: the tubules are mostly open and the peritubular dentin can be observed in the cross-section. At the periphery of the treated area (figure 3(b)), corresponding to the lower values of irradiance, the tubules are concealed by this porous layer.
The dentinal structure is less evident in the surface obtained by a laser treatment with 3 J cm−2 (figures 4(a) and (b)). The surface is irregular in appearance and porous. It seems to be formed of very small, unmelted, angular particles aggregated in large irregularly shaped clusters, forming a crust, similarly to what is observed in the central region of surfaces treated with 1.8 J cm−2. However, for this higher fluence a relatively large number of irregularly shaped ablation debris, as well as a few spherical particles, are observed on the surface. As a result of this complex surface topography, the overall surface roughness is larger than for lower fluences. The dentinal tubules are also less evident because they tend to be receded in relation to the surface.
Figure 4. (a), (b) SEM images of a large area dentin surface after laser treatment with 3 J cm−2. The white arrow in (b) shows a spherical particle. (c) SEM micrograph of the transverse cross-section across a laser-treated dentin surface.
Download figure:
Standard imageIndependently of the fluence used no significant cracking was observed below the laser-treated surface (figure 4(c)), in contrast with the observations of Cardoso et al [20, 27] for dentin ablated with a Er,Cr : YSGG laser in certain parameter ranges.
3.3. Surface composition
To investigate the chemical changes due to the laser treatment, untreated and laser-treated surfaces were analysed by XPS, XRD and FTIR spectroscopy. The relevant regions of the XPS spectra acquired from the untreated and treated samples (curves 1 and 2, respectively) are presented in figure 5. The C 1s carbon peak in both spectra (figure 5(a)) can be deconvolved into two peaks. The first one, corresponding to a binding energy of 285 eV, is due to surface contamination by hydrocarbons, while the other one, located at about 287.5 eV, can be attributed to the organic compounds present in dentin, in particular type I collagen [28]. The relative amplitude of these peaks does not change significantly due to the laser treatment. The same observation is valid for the N 1s nitrogen peak at 400 eV (figure 5(b)), which is associated with the amide groups of type I collagen molecules. Finally, the peaks in the Ca 2p and P 2p regions (figures 5(c) and (d)), corresponding to hydroxyapatite, remain unaltered after the laser treatment. In conclusion, the XPS results show that the organic and inorganic matter is ablated uniformly from the surface, a conclusion that is demonstrated by the fact that the Ca/N ratio is identical within the limits of experimental error (∼1.9 ± 0.1) for both laser-treated and untreated surfaces.
Figure 5. Relevant regions of the XPS spectra of untreated (curve 1) and laser-treated (curve 2) dentin sample: (a) C 1s; (b) N 1s; (c) Ca 2p and (d) P 2p.
Download figure:
Standard imageThe FTIR spectra of dentin under the untreated condition and after laser treatment with an average fluence of 1 J cm−2 are depicted in figures 6(a) and (b), respectively. They are similar to the infrared spectra of dentin samples prepared by standard mechanical cutting and polishing techniques [29]. The phosphate and carbonate absorption bands in these spectra are associated with the mineral phase of dentin, a calcium deficient carbonated apatite [30]. The most intense peaks associated with
 appear at about 1045 and 964 cm−1 and correspond to the ν3 antisymmetric PO stretching mode and the ν1 symmetric stretching mode, respectively [2, 31]. The absorption bands at 1437 cm−1 and 1456 cm−1 can be attributed to the ν3 mode of
appear at about 1045 and 964 cm−1 and correspond to the ν3 antisymmetric PO stretching mode and the ν1 symmetric stretching mode, respectively [2, 31]. The absorption bands at 1437 cm−1 and 1456 cm−1 can be attributed to the ν3 mode of
 substituted in B-type
substituted in B-type
 sites and A-type OH− anionic sites, respectively [32]. The band at 872 cm−1 is due to ν2 mode
sites and A-type OH− anionic sites, respectively [32]. The band at 872 cm−1 is due to ν2 mode
 [33].
[33].
Figure 6. Infrared spectra of untreated (a) and laser-treated (b) dentin samples.
Download figure:
Standard imageThe bands present in the spectra between 1650 and 1200 cm−1 can be ascribed to the amide group of collagen, the main organic component of dentin. The bands at 1647 cm−1, 1541 cm−1 and 1227 cm−1 are also due to the amide I (C–O bond), amide II (C–N stretching and N–H deformation modes) and amide III groups of the collagen molecule, respectively. The broad absorption band centred at 3320 cm−1 can be attributed to the amide A (N–H stretching mode) of collagen. The peaks at 2920 and 2850 cm−1 correspond to C–H bonds of organic compounds in general, including surface contaminants [29, 33, 34]. After laser ablation, changes in the structure of the band corresponding to C–H bonds in the organic compounds were observed. However, the position and relative amplitude of the other IR absorption peaks are not significantly affected by the laser treatment.
The x-ray diffractogram of dentin prior to laser treatment is shown in figure 7(a). All the diffraction peaks observed in this diffractogram can be indexed as a nonstoichiometric hydroxyapatite [35], according to ICDD card number 09-0432. In figures 7(b) and (c), the XRD patterns of the untreated and laser-treated dentin samples with 1 and 3 J cm−2 are compared. The only difference between the diffractograms is a slight widening of the peaks in the region 46° < 2θ < 57° for the sample treated with 3 J cm−2. No peaks of high temperature calcium phosphates, such as α and β tricalcium phosphate, are observed.
Figure 7. X-ray diffractograms of (a) untreated dentin sample, (b) sample treated with 1 J cm−2, and (c) sample treated with 3 J cm−2.
Download figure:
Standard image4. Discussion
The ablation threshold of dentin under the experimental conditions used (radiation wavelength 1030 nm, pulse duration 500 fs, pulse repetition rate 1 kHz) is 0.6 ± 0.2 J cm−2. The ablation rate depends on the radiation fluence and on the beam-scanning mode, but is in the range 1–2 µm/pulse for fluences of about 3 J cm−2. The value of the ablation threshold found in this work is larger than the value found by Kruger et al [22] (0.3 J cm−2 for dentin and 0.6 J cm−2 for enamel) and in good agreement with the value determined by Neev et al [24] using a laser with 350 fs pulse duration and 1050 nm wavelength (0.5 J cm−2 for dentin and 0.7 J cm−2 for enamel). It is, however, substantially lower than the value determined by Rode et al [23] for either a 95 fs pulse duration and 805 nm radiation wavelength laser or a laser with 150 fs pulse duration and 780 nm radiation wavelength (2.2 ± 0.1 J cm−2). On the other hand, the dentin ablation rate measured in this work is, for the same radiation fluence, similar to the one found by Neev et al [24] and larger than the ablation rates found by Rode et al [23] by about one order of magnitude.
The ablation of dentin with femtosecond laser radiation leads to the formation of surfaces with no traces of surface melting, deformation or carbonization. The smear layer is entirely removed by the laser treatment and the dentinal tubules remain open. For fluences only slightly higher than the ablation threshold the morphology of the laser-treated surfaces is very similar to the fracture surfaces observed by Imbeni et al [36]. These surfaces resulted from a brittle fracture mechanism initiated by an atomically sharp stress concentrator (a fatigue crack), with the fracture surface propagating perpendicularly to the dentinal tubules. Rasmussen and Patchin [37] studied the fracture mechanism of human dentin and found that dentin is anisotropic with respect to mechanical failure, the easiest fracture plane being perpendicular to the tubules. This observation was confirmed by later authors, who showed that the ultimate tensile strength [38] and the fracture toughness [39] of dentin is lower when the tubules are perpendicular to the maximum stress direction than when they are parallel to this direction. The morphology of the surface depicted in figures 2(b) and (c) is consistent with these observations, and suggest that the surface resulting from laser ablation under these conditions result from a brittle fracture mechanism. It is also interesting to remark that the cracks in the peritubular dentin sheets observed in figure 2(d) of this paper are very similar to the cracks observed in figure 8 of [36], which the authors considered as an indication that brittle fracture of dentin would be initiated in peritubular dentin and propagate into intertubular dentin. An important fact is that no sub-superficial cracking was observed in any of the samples studied, so it can be assumed that femtosecond laser ablation will not degrade the cohesive strength of dentin restoration systems, in contrast to ablation with Er,Cr : YSGG laser within certain parameter ranges, as found by Cardoso et al [20, 27]. Increasing the fluence leads to a more complex surface morphology, where weakly agglomerated ablation debris partially conceals the dentin structure. Some resolidified mineral droplets are observed, more so at the higher fluences.
In terms of surface structure and chemical composition, XRD, XPS and FTIR analyses showed that the laser treatment does not induce significant changes in the constitution and chemical composition of dentin. In contrarst to what was observed after laser treatment with KrF laser radiation (λ = 248 nm, 30 ns pulse duration, fluences in the 1–20 J cm−2 range) [14], the organic matter is not preferentially removed from the surface as demonstrated by the similarity of the relative amplitude of the nitrogen peak at 400.0 eV in the XPS spectra of the untreated and laser-treated samples, associated with type I collagen amide groups. No traces of high temperature phosphates, such as the β-tricalcium phosphate, were observed either, as was the case after laser treatment with a Nd : YAG laser [40].
The similarity of the morphology of the laser-treated dentin surfaces and of fracture surfaces resulting from tensile testing and the lack of significant changes of the surface structure and chemical composition are certainly related to the femtosecond laser ablation mechanisms of dentin. Dentin is a composite material consisting of collagen fibres reinforced with hydroxyapatite nanocrystals and impregnated with water. However, the structure of dentin is not uniform. It presents channels or tubules a few micrometres in diameter irradiating from the dentinal pulp to the dentin/enamel interface. These tubules are surrounded by a cuff of peritubular dentin, the remaining of the tooth bulk being formed of intertubular dentin. Intertubular dentin is composed of a network of collagen fibres with dispersed nanocrystals of apatite. Although apatite is the predominant phase (about 70 wt%), the continuous phase (matrix) is collagen. By contrast, peritubular dentin consists essentially of mineral phase with embedded collagen fibres. In a precedent paper [14] the authors showed that 248 nm wavelength laser radiation being weakly absorbed by water, the ablation behaviour of dentin submitted to intense radiation of this wavelength is controlled by the electronic structure and optical properties of its two main solid constituents, collagen and hydroxyapatite. Since the energy of 248 nm photons is higher than the energy of the covalent bonds in collagen molecules, radiation is absorbed by valence electrons in the collagen molecules, leading to their photochemical disruption. On the contrary, the energy of those photons is lower than the energy gap of hydroxyapatite crystals. The absorption coefficient of hydroxyapatite for this wavelength is relatively low and radiation is mainly absorbed by electrons associated with surfaces and crystallographic defects in the hydroxyapatite crystals. As a result, for fluences lower than 1 J cm−2 and when the dentinal tubules are perpendicular to the treated surface, a differential ablation behaviour develops, leading to preferential ablation of intertubular dentin as compared with peritubular dentin and to the development of rough surface topographies, characterized by cones formed of partially melted peritubular dentin [15] protruding from the average surface, consisting of intertubular dentin. When the dentinal tubules are tilted in relation to the surface, the ablation behaviour changes and a flat surface develops due to the shadowing of peritubular dentin by peritubular dentin sheets. For fluences higher than 1 J cm−2 the ablation rates of intertubular and peritubular dentin are similar and both types of dentin are uniformly removed.
Rode et al [23] suggested that, depending on the laser radiation intensity and pulse duration, ablation of dentin by sub-picosecond laser pulses may occur by thermal or non-thermal mechanisms. If the intensity exceeds 1013–1014 W cm−2, complete ionization of the target material occurs during the first stages of the laser pulses. The free electrons generated by this initial ionization process interact with the atoms in the target and ionize them as well, leading to the formation of a very thin layer of plasma at the surface of the sample. This plasma absorbs all the incoming laser radiation, thus acting as a barrier and preventing the laser radiation to reach and be directly absorbed by the underlying target material. If the energy absorbed by the plasma exceeds the sum of the first ionization potential and the energy required to leave the solid, the electrons will escape the target, leaving behind a layer of positively charged ions that are subsequently pulled out of the target by the repulsive forces between these positive charges. This ablation mechanism is called electrostatic ablation. It is a non-thermal process, which does not involve melting or evaporation in a conventional sense. Furthermore, since ionization at these high radiation intensities is essentially independent of the target material, the ablation mechanism and the ablation rate of all dentin constituents should be similar. For lower intensities, the excited electrons will transfer their excess energy to the lattice in a time scale longer than the laser pulse duration and the ablation mechanism is mainly thermal. Ablation occurs after the laser pulse, in a time scale similar to ablation with nanosecond pulse duration lasers. In this work, 500 fs laser pulses with energy between 0.5 and 1 mJ were focused onto the dentin surface in a focal spot of about 100 µm diameter, providing a radiation intensity between 1.3 and 2.5 × 1013 W cm−2. For these intensities, the ablation mechanism is expected to be predominantly electrostatic, which explains the similarity of the morphology of the laser-treated dentin surfaces and of fracture surfaces resulting from tensile testing, the similarity of the ablation rates of intertubular and peritubular dentin and that no preferential ablation of collagen occurs, as revealed by the XPS and FTIR results. The non-thermal nature of this ablation process also explains the lack of significant traces of melting, thermal cracking or carbonization and the absence of high-temperature phosphate phases in the laser-treated surfaces.
The surface resulting from treatment with femtosecond lasers should be favourable to standard bonding procedures because it presents a micro-retentive irregular topography, free of smear layer and with open dentinal tubules. Moreover, since the organic components of dentin are only minimally affected by the laser treatment, bonding between dentin and the restoration materials can be accomplished not only by the penetration of the bonding material into the dentinal tubules to provide mechanical retention via 'resin tags', but also by the interaction of resin with the collagen network to form a hybrid layer [41] of fundamental importance to ensure a perfect bonding. However, adhesion studies are needed to evaluate the bonding behaviour of dentin treated with femtosecond pulse duration lasers to standard restorative materials.
5. Conclusions
- (1)The average dentin ablation threshold for a pulse duration of 500 fs and radiation wavelength 1030 nm is 0.6 ± 0.2 J cm−2. The ablation rates achieved depend on the surface treatment strategy but are typically in the range 1–2 µm/pulse for an average fluence of about 3 J cm−2.
- (2)The ablation surfaces present an irregular and rugged appearance but no traces of surface melting, deformation, cracking or carbonization are observed. The smear layer was entirely removed by the laser treatment. For fluences only slightly higher than the ablation threshold the morphology of the laser-treated surfaces was very similar to the dentin fracture surfaces and the dentinal tubules remain open. For higher fluences the surface was more porous and the dentin structure was partially concealed by ablation debris and a few resolidified droplets.
- (3)No sub-superficial cracking was observed, independently on the laser processing parameters and laser processing method used.
- (4)The dentin constitution and chemical composition were not significantly modified by the laser treatment in the processing parameter range used. The organic matter was not preferentially removed from the surface and no traces of high temperature phosphates, such as the β-tricalcium phosphate, were observed.
- (5)The results obtained are compatible with an electrostatic ablation mechanism.
Acknowledgments
This work was financially supported by Fundação para a Ciência e Tecnologia (FCT) under de project 'Minimally invasive dental care based on femtosecond laser technology' (Ref. PTDC/SAU-BEB/67141/2006).