Abstract
Photochemical internalization (PCI) enhances adenovirus (Ad) transgene expression in a variety of cell lines in vitro. However, measurements of the photochemical effect on transduction in multicellular environments are lacking. In this study, spheroids of DU 145 prostate cancer cells were used as a model to evaluate Ad serotype 5 (Ad5) transduction in a multicellular environment in response to PCI treatment. Furthermore, the Ad5 was coated with poly(2-methyl-acrylic acid 2-[(2-(dimethylamino)-ethyl)-methyl-amino]-ethyl ester) (pDAMA) to evaluate whether physicochemical properties such as charge and size of viral vectors affect transduction of photochemically treated spheroids.
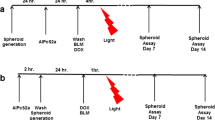
Spheroids incubated with photosensitizer TPPS2a (1 µg ml−1) and infected with adenovirus contained 3-fold higher percentage of reporter gene expressing cells after exposure to blue light (0.42 J cm−2) compared to no light, as analysed by flow cytometry of dissociated spheroids two days after treatment. The cells within the infected spheroids were further divided into three sections corresponding to the interior, intermediate and peripheral layers of the spheroids. This was performed by staining the spheroids with a diffusion-limited dye prior to dissociation. Transduction of cells within photochemically treated and untreated spheroids was heterogeneous, with a radial reduction of transgene expression towards the inner section of the spheroid. The coating of Ad with pDAMA induced up to 2-fold decrease in transduction of cells in the interior section of spheroids compared to uncomplexed Ad, while transduction of the peripheral section remained unchanged. The decrease in transduction could be related to reduced diffusion due to the size of the Ad—pDAMA complexes.
Similar content being viewed by others
References
K. Berg, P. K. Selbo, L. Prasmickaite, T. E. Tjelle, K. Sandvig, J. Moan, G. Gaudernack, O. Fodstad, S. Kjølsrud, H. Anholt, G. H. Rodal, S. K. Rodal, A. Høgset, Photochemical internalization: a novel technology for delivery of macromolecules into cytosol, Cancer Res., 1999, 59, 1180–1183.
B. W. Henderson and T. J. Dougherty, How does photodynamic therapy work?, Photochem. Photobiol., 1992, 55, 145–157.
K. Berg and J. Moan, Lysosomes as photochemical targets, Int. J. Cancer, 1994, 59, 814–822.
A. Høgset, L. Prasmickaite, T. E. Tjelle and K. Berg, Photochemical transfection: a new technology for light-induced, site-directed gene delivery, Hum. Gene Ther., 2000, 11, 869–880.
A. Høgset, B. Ø. Engesæter, L. Prasmickaite, K. Berg, O. Fodstad, G. M. Mælandsmo, Light-induced adenovirus gene transfer, an efficient and specific gene delivery technology for cancer gene therapy, Cancer Gene Ther., 2002, 9, 365–371.
A. Bonsted, A. Høgset, F. Hoover and K. Berg, Photochemical enhancement of gene delivery to glioblastoma cells is dependent on the vector applied, Anticancer Res., 2005, 25, 291–297.
L. Prasmickaite, A. Høgset, B. Ø. Engesæter, A. Bonsted and K. Berg, Light-directed gene delivery by photochemical internalisation, Expert Opin. Biol. Ther., 2004, 4, 1403–1412.
B. Ø. Engesæter, S. Tveito, A. Bonsted, O. Engebraaten, K. Berg, G. M. Mælandsmo, Photochemical treatment with endosomally localized photosensitizers enhances the number of adenoviruses in the nucleus, J. Gene Med., 2006, in press.
R. Tomanin and M. Scarpa, Why do we need new gene therapy viral vectors? Characteristics, limitations and future perspectives of viral vector transduction, Curr. Gene Ther., 2004, 4, 357–372.
U. F. Greber, M. Willetts, P. Webster and A. Helenius, Stepwise dismantling of adenovirus 2 during entry into cells, Cell, 1993, 75, 477–486.
P. L. Leopold, B. Ferris, I. Grinberg, S. Worgall, N. R. Hackett and R. G. Crystal, Fluorescent virions: dynamic tracking of the pathway of adenoviral gene transfer vectors in living cells, Hum. Gene Ther., 1998, 9, 367–378.
B. Ø. Engesæter, A. Bonsted, K. Berg, A. Høgset, O. Engebraten, O. Fodstad, D. T. Curiel, G. M. Mælansdmo, PCI-enhanced adenoviral transduction employs the known uptake mechanism of adenoviral particles, Cancer Gene Ther., 2005, 12, 439–448.
R. M. Sutherland, Cell and environment interactions in tumor microregions: the multicell spheroid model, Science, 1988, 240, 177–184.
N. S. Waleh, J. Gallo, T. D. Grant, B. J. Murphy, R. H. Kramer and R. M. Sutherland, Selective down-regulation of integrin receptors in spheroids of squamous cell carcinoma, Cancer Res., 1994, 54, 838–843.
T. Nederman, B. Norling, B. Glimelius, J. Carlsson and U. Brunk, Demonstration of an extracellular matrix in multicellular tumor spheroids, Cancer Res., 1984, 44, 3090–3097.
A. Fasbender, J. Zabner, M. Chillon, T. O. Moninger, A. P. Puga, B. L. Davidson and M. J. Welsh, Complexes of adenovirus with polycationic polymers and cationic lipids increase the efficiency of gene transfer in vitro and in vivo, J. Biol. Chem., 1997, 272, 6479–6489.
J. M. Kaplan, S. E. St Pennington, J. A. George, L. A. Woodworth, A. Fasbender, J. Marshall, S. H. Cheng, S. C. Wadsworth, R. J. Gregory and A. E. Smith, Potentiation of gene transfer to the mouse lung by complexes of adenovirus vector and polycations improves therapeutic potential, Hum. Gene Ther., 1998, 9, 1469–1479.
E. Dodds, T. A. Piper, S. J. Murphy and G. Dickson, Cationic lipids and polymers are able to enhance adenoviral infection of cultured mouse myotubes, J. Neurochem., 1999, 72, 2105–2112.
A. Bonsted, B. Ø. Engesæter, A. Høgset, G. M. Mælandsmo, L. Prasmickaite, O. Kaalhus and K. Berg, Transgene expression is increased by photochemically mediated transduction of polycation-complexed adenoviruses, Gene Ther., 2004, 11, 152–160.
A. Bonsted, B. Ø. Engesæter, A. Høgset, G. M. Mælandsmo, L. Prasmickaite, C. D’Oliveira, W. E. Hennink, J. H. van Steenis and K. Berg, Photochemically enhanced transduction of polymer-complexed adenovirus targeted to the epidermal growth factor receptor, J. Gene Med., 2005, 8, 286–297.
H. E. Davis, M. Rosinski, J. R. Morgan and M. L. Yarmush, Charged polymers modulate retrovirus transduction via membrane charge neutralization and virus aggregation, Biophys. J., 2004, 86, 1234–1242.
R. K. Jain, Transport of molecules in the tumor interstitium: a review, Cancer Res., 1987, 47, 3039–3051.
L. A. Wenning and R. M. Murphy, Coupled cellular trafficking and diffusional limitations in delivery of immunotoxins to multicell tumor spheroids, Biotechnol. Bioeng., 1999, 62, 562–575.
G. Fracasso and M. Colombatti, Effect of therapeutic macromolecules in spheroids, Crit. Rev. Oncol. Hematol., 2000, 36, 159–178.
B. Ríhová, J. Strohalm, J. Prausova, K. Kubackova, M. Jelinkova, L. Rozprimova, M. Sirova, D. Plocova, T. Etrych, V. Subr, T. Mrkvan, M. Kovar and K. Ulbrich, Cytostatic and immunomobilizing activities of polymer-bound drugs: experimental and first clinical data, J. Controlled Release, 2003, 91, 1–16.
R. R. Palmer, A. L. Lewis, L. C. Kirkwood, S. F. Rose, A. W. Lloyd, T. A. Vick and P. W. Stratford, Biological evaluation and drug delivery application of cationically modified phospholipid polymers, Biomaterials, 2004, 25, 4785–4796.
A. M. Funhoff, C. F. van Nostrum, G. A. Koning, N. M. Schuurmans-Nieuwenbroek, D. J. Crommelin and W. E. Hennink, Endosomal escape of polymeric gene delivery complexes is not always enhanced by polymers buffering at low pH, Biomacromolecules, 2004, 5, 32–39.
J. Y. Cherng, P. van de Wetering, H. Talsma, D. J. Crommelin and W. E. Hennink, Effect of size and serum proteins on transfection efficiency of poly((2-dimethylamino)ethyl methacrylate)-plasmid nanoparticles, Pharm. Res., 1996, 13, 1038–1042.
M. Hitt, A. J. Bett, C. L. Addison, L. Prevec and F. L. Graham, Techniques for human adenovirus vector construction and characterization In Methods in Molecular Genetics, ed. K. W. Adolph, Academic Press, New York, 1995, pp. 13–30.
T. Mosmann, Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays, J. Immunol. Methods, 1983, 65, 55–63.
R. E. Durand, Use of Hoechst 33342 for cell selection from multicell systems, J. Histochem. Cytochem., 1982, 30, 117–122.
P. J. Canatella, M. M. Black, D. M. Bonnichsen, C. McKenna and M. R. Prausnitz, Tissue electroporation: quantification and analysis of heterogeneous transport in multicellular environments, Biophys. J., 2004, 86, 3260–3268.
C. P. Winsor, The Gompertz curve as a growth curve, Proc. Natl. Acad. Sci. U. S. A., 1932, 18, 1–7.
L. Prasmickaite, A. Høgset, P. K. Selbo, B. Ø. Engesæter, M. Hellum and K. Berg, Photochemical disruption of endocytic vesicles before delivery of drugs: a new strategy for cancer therapy, Br. J. Cancer, 2002, 86, 652–657.
T. Bouvier, M. Troussellier, A. Anzil, C. Courties and P. Servais, Using light scatter signal to estimate bacterial biovolume by flow cytometry, Cytometry, 2001, 44, 188–194.
H. M. Shapiro, Practical flow cytometry, Wiley-Liss, New York, 4th edn, 2003.
M. Essand, C. Gronvik, T. Hartman and J. Carlsson, Radioimmunotherapy of prostatic adenocarcinomas: effects of 131I-labelled E4 antibodies on cells at different depth in DU 145 spheroids, Int. J. Cancer, 1995, 63, 387–394.
P. J. Canatella, J. F. Karr, J. A. Petros and M. R. Prausnitz, Quantitative study of electroporation-mediated molecular uptake and cell viability, Biophys. J., 2001, 80, 755–764.
C. M. West and J. V. Moore, Flow cytometric analysis of intracellular hematoporphyrin derivative in human tumor cells and multicellular spheroids, Photochem. Photobiol., 1989, 50, 665–669.
M. Juweid, R. Neumann, C. Paik, M. J. Perez-Bacete, J. Sato, W. van Osdol and J. N. Weinstein, Micropharmacology of monoclonal antibodies in solid tumors: direct experimental evidence for a binding site barrier, Cancer Res., 1992, 52, 5144–5153.
J. Moan, K. Berg and V. Iani, Action spectra of dyes relevant for photodynamic therapy, in Photodynamic Tumor Therapy, ed. J. G. Moser, Harwood Publishers, London, 1998, pp. 169–181.
M. G. Nichols and T. H. Foster, Oxygen diffusion and reaction kinetics in the photodynamic therapy of multicell tumour spheroids, Phys. Med. Biol., 1994, 39, 2161–2181.
A. M. Funhoff, C. F. van Nostrum, M. C. Lok, J. A. Kruijtzer, D. J. Crommelin and W. E. Hennink, Cationic polymethacrylates with covalently linked membrane destabilizing peptides as gene delivery vectors, J. Controlled Release, 2005, 101, 233–246.
H. R. Shen, J. D. Spikes, P. Kopecekova and J. Kopecek, Photodynamic crosslinking of proteins. I. Model studies using histidine- and lysine-containing N-(2-hydroxypropyl)methacrylamide copolymers, J. Photochem. Photobiol., 1996, 34, 203–210.
P. K. Selbo, G. Sivam, O. Fodstad, K. Sandvig and K. Berg, In vivo documentation of photochemical internalization, a novel approach to site specific cancer therapy, Int. J. Cancer, 2001, 92, 761–766.
A. Dietze, Q. Peng, P. K. Selbo, O. Kaalhus, C. Muller, S. Bown and K. Berg, Enhanced photodynamic destruction of a transplantable fibrosarcoma using photochemical internalisation of gelonin, Br. J. Cancer, 2005, 92, 2004–2009.
C. H. Sibata, V. C. Colussi, N. L. Oleinick and T. J. Kinsella, Photodynamic therapy in oncology, Expert Opin. Pharmacother., 2001, 2, 917–927.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonsted, A., Engesæter, B.Ø., Høgset, A. et al. Photochemically enhanced adenoviral transduction in a multicellular environment. Photochem Photobiol Sci 5, 411–421 (2006). https://doi.org/10.1039/b515066c
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/b515066c