Abstract
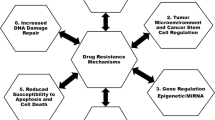
Resistance to chemotherapy, molecular targeted therapy as well as radiation therapy is a major obstacle for cancer treatment. Cancer resistance may be exerted through multiple different mechanisms which may be orchestrated as observed in multidrug resistance (MDR). Cancer resistance may be intrinsic or acquired and often leaves patients without any treatment options. Strategies for alternative treatment modalities for resistant cancer are therefore highly warranted. Photochemical internalization (PCI) is a technology for cytosolic delivery of macromolecular therapeutics based on the principles of photodynamic therapy (PDT). The present report reviews the current knowledge of PCI of therapy-resistant cancers. In summary, PCI may be able to circumvent several of the major mechanisms associated with resistance towards chemotherapeutics including increased expression of drug efflux pumps, altered intracellular drug distribution and increased ROS scavenging. Current data also suggest PCI of targeted toxins as highly effective in cancers resistant to clinically available targeted therapy such as monoclonal antibodies (mAbs) and tyrosine kinase inhibitors (TKIs). PCI may therefore, in general, represent a future treatment option for cancers resistant to other therapies.
Similar content being viewed by others
References
M. R. Lackner, T. R. Wilson, J. Settleman, Mechanisms of acquired resistance to targeted cancer therapies, Future Oncol., 2012, 8, 999–1014.
P. Ramos, M. Bentires-Alj, Mechanism-based cancer therapy: resistance to therapy, therapy for resistance, Oncogene, 2014, 10, 10.1038/onc.2014.314
G. Housman, S. Byler, S. Heerboth, K. Lapinska, M. Longacre, N. Snyder, et al., Drug resistance in cancer: an overview, Cancers, 2014, 6, 1769–1792.
C. Holohan, S. S. Van, D. B. Longley, P. G. Johnston, Cancer drug resistance: an evolving paradigm, Nat. Rev. Cancer, 2013, 13, 714–726.
T. Ozben, Mechanisms and strategies to overcome multiple drug resistance in cancer, FEBS Lett., 2006, 580, 2903–2909.
Z. H. Siddik, Cisplatin: mode of cytotoxic action and molecular basis of resistance, Oncogene, 2003, 22, 7265–7279.
K. V. Kosuri, X. Wu, L. Wang, M. A. Villalona-Calero, G. A. Otterson, An epigenetic mechanism for capecitabine resistance in mesothelioma, Biochem. Biophys. Res. Commun., 2010, 391, 1465–1470.
J. F. Apperley, Part I: mechanisms of resistance to imatinib in chronic myeloid leukaemia, Lancet Oncol., 2007, 8, 1018–1029.
P. Nagy, E. Friedlander, M. Tanner, A. I. Kapanen, K. L. Carraway, J. Isola, et al., Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line, Cancer Res., 2005, 65, 473–482.
A. Carracedo, L. Ma, J. Teruya-Feldstein, F. Rojo, L. Salmena, A. Alimonti, et al., Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer, J. Clin. Invest., 2008, 118, 3065–3074.
Y. Pommier, O. Sordet, S. Antony, R. L. Hayward, K. W. Kohn, Apoptosis defects and chemotherapy resistance: molecular interaction maps and networks, Oncogene, 2004, 23, 2934–2949.
L. Lundholm, P. Haag, D. Zong, T. Juntti, B. Mork, R. Lewensohn, et al., Resistance to DNA-damaging treatment in non-small cell lung cancer tumor-initiating cells involves reduced DNA-PK/ATM activation and diminished cell cycle arrest, Cell Death Dis., 2013, 4, e478–e486.
S. Kamesaki, H. Kamesaki, T. J. Jorgensen, A. Tanizawa, Y. Pommier, J. Cossman, bcl-2 protein inhibits etoposide-induced apoptosis through its effects on events subsequent to topoisomerase II-induced DNA strand breaks and their repair, Cancer Res., 1993, 53, 4251–4256.
M. I. Walton, D. Whysong, P. M. O’Connor, D. Hockenbery, S. J. Korsmeyer, K. W. Kohn, Constitutive expression of human Bcl-2 modulates nitrogen mustard and camptothecin induced apoptosis, Cancer Res., 1993, 53, 1853–1861.
B. C. Baguley, Multiple drug resistance mechanisms in cancer, Mol. Biotechnol., 2010, 46, 308–316.
A. Marusyk, K. Polyak, Tumor heterogeneity: causes and consequences, Biochim. Biophys. Acta, 2010, 1805, 105–117.
M. Karvela, G. V. Helgason, T. L. Holyoake, Mechanisms and novel approaches in overriding tyrosine kinase inhibitor resistance in chronic myeloid leukemia, Expert Rev. Anticancer Ther., 2012, 12, 381–392.
V. Nardi, M. Azam, G. Q. Daley, Mechanisms and implications of imatinib resistance mutations in BCR-ABL, Curr. Opin. Hematol., 2004, 11, 35–43.
A. Quintas-Cardama, E. J. Jabbour, Considerations for early switch to nilotinib or dasatinib in patients with chronic myeloid leukemia with inadequate response to first-line imatinib, Leuk. Res., 2013, 37, 487–495.
G. Kibria, H. Hatakeyama, H. Harashima, Cancer multidrug resistance: mechanisms involved and strategies for circumvention using a drug delivery system, Arch. Pharm. Res., 2014, 37, 4–15.
K. Berg, P. K. Selbo, L. Prasmickaite, T. E. Tjelle, K. Sandvig, J. Moan, et al., Photochemical internalization: a novel technology for delivery of macromolecules into cytosol, Cancer Res., 1999, 59, 1180–1183.
P. K. Selbo, A. Weyergang, A. Hogset, O. J. Norum, M. B. Berstad, M. Vikdal, et al., Photochemical internalization provides time- and space-controlled endolysosomal escape of therapeutic molecules, J. Controlled Release, 2010, 148, 2–12.
K. Berg, S. Nordstrand, P. K. Selbo, D. T. Tran, E. Ngell-Petersen, A. Hogset, Disulfonated tetraphenyl chlorin (TPCS2a), a novel photosensitizer developed for clinical utilization of photochemical internalization, Photochem. Photobiol. Sci., 2011, 10, 1637–1651.
P. Agostinis, K. Berg, K. A. Cengel, T. H. Foster, A. W. Girotti, S. O. Gollnick, et al., Photodynamic therapy of cancer: An update, CA-Cancer J. Clin., 2011, 61, 250–281.
A. Dietze, Q. Peng, P. K. Selbo, O. Kaalhus, C. Muller, S. Bown, et al., Enhanced photodynamic destruction of a transplantable fibrosarcoma using photochemical internalisation of gelonin, Br. J. Cancer, 2005, 92, 2004–2009.
M. B. Berstad, L. H. Cheung, K. Berg, Q. Peng, A. S. Fremstedal, S. Patzke, et al., Design of an EGFR-targeting toxin for photochemical delivery: in vitro and in vivo selectivity and efficacy, Oncogene, 2015, 10, 10.1038/onc.2015.15
O. J. Norum, J. V. Gaustad, E. ngell-Petersen, E. K. Rofstad, Q. Peng, K. E. Giercksky, et al., Photochemical internalization of bleomycin is superior to photodynamic therapy due to the therapeutic effect in the tumor periphery, Photochem. Photobiol., 2009, 85, 740–749.
M. Hakerud, P. K. Selbo, Y. Waeckerle-Men, E. Contassot, P. Dziunycz, T. M. Kundig, et al., Photosensitisation facilitates cross-priming of adjuvant-free protein vaccines and stimulation of tumour-suppressing CD8 T cells, J. Controlled Release, 2015, 198, 10–17.
M. Hakerud, Y. Waeckerle-Men, P. K. Selbo, T. M. Kundig, A. Hogset, P. Johansen, Intradermal photosensitisation facilitates stimulation of MHC class-I restricted CD8 T-cell responses of co-administered antigen, J. Controlled Release, 2014, 174, 143–150.
K. Svanberg, N. Bendsoe, J. Axelsson, S. Andersson-Engels, S. Svanberg, Photodynamic therapy: superficial and interstitial illumination, J. Biomed. Opt., 2010, 15, 041502.
K. Berg, A. Dietze, O. Kaalhus, A. Hogset, Site-specific drug delivery by photochemical internalization enhances the antitumor effect of bleomycin, Clin. Cancer Res., 2005, 11, 8476–8485.
J. M. Vergnon, R. M. Huber, K. Moghissi, Place of cryotherapy, brachytherapy and photodynamic therapy in therapeutic bronchoscopy of lung cancers, Eur. Respir. J., 2006, 28, 200–218.
T. Yano, K. Hatogai, H. Morimoto, Y. Yoda, K. Kaneko, Photodynamic therapy for esophageal cancer, Ann. Transl. Med., 2014, 2, 29–5839.
D. Bechet, S. R. Mordon, F. Guillemin, M. A. Barberi-Heyob, Photodynamic therapy of malignant brain tumours: a complementary approach to conventional therapies, Cancer Treat Rev., 2014, 40, 229–241.
O. J. Norum, K. E. Giercksky, K. Berg, Photochemical internalization as an adjunct to marginal surgery in a human sarcoma model, Photochem. Photobiol. Sci., 2009, 8, 758–762.
D. W. Pack, A. S. Hoffman, S. Pun, P. S. Stayton, Design and development of polymers for gene delivery, Nat. Rev. Drug Discovery, 2005, 4, 581–593.
V. P. Torchilin, Recent advances with liposomes as pharmaceutical carriers, Nat. Rev. Drug Discovery, 2005, 4, 145–160.
M. Wu, Enhancement of immunotoxin activity using chemical and biological reagents, Br. J. Cancer, 1997, 75, 1347–1355.
H. Fuchs, D. Bachran, H. Panjideh, N. Schellmann, A. Weng, M. F. Melzig, et al., Saponins as tool for improved targeted tumor therapies, Curr. Drug Targets, 2009, 10, 140–151.
E. Bossu, O. Amar, R. M. Parache, D. Notter, P. Labrude, C. Vigneron, et al., Determination of the maximal tumor/normal skin ratio after HpD or m-THPC administration in hairless mouse (SKh-1) by fluorescence spectroscopy—a non-invasive method, Anticancer Drugs, 1997, 8, 67–72.
W. L. Yip, A. Weyergang, K. Berg, H. H. Tonnesen, P. K. Selbo, Targeted delivery and enhanced cytotoxicity of cetuximab-saporin by photochemical internalization in EGFR-positive cancer cells, Mol. Pharm., 2007, 4, 241–251.
Z. Bikadi, I. Hazai, D. Malik, K. Jemnitz, Z. Veres, P. Hari, et al., Predicting P-glycoprotein-mediated drug transport based on support vector machine and three-dimensional crystal structure of P-glycoprotein, PLoS One, 2011, 6, e25815.
K. Ueda, Y. Taguchi, M. Morishima, How does P-glycoprotein recognize its substrates?, Semin. Cancer Biol., 1997, 8, 151–159.
S. Mayor, R. E. Pagano, Pathways of clathrin-independent endocytosis, Nat. Rev. Mol. Cell Biol., 2007, 8, 603–612.
P. K. Selbo, A. Weyergang, A. Bonsted, S. G. Bown, K. Berg, Photochemical internalization of therapeutic macromolecular agents: a novel strategy to kill multidrug-resistant cancer cells, J. Pharmacol. Exp. Ther., 2006, 319, 604–612.
J. W. Jonker, M. Buitelaar, E. Wagenaar, M. A. Van Der Valk, G. L. Scheffer, R. J. Scheper, et al., The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 15649–15654.
R. W. Robey, K. Steadman, O. Polgar, S. E. Bates, ABCG2-mediated transport of photosensitizers: potential impact on photodynamic therapy, Cancer Biol. Ther., 2005, 4, 187–194.
P. Krishnamurthy, D. D. Ross, T. Nakanishi, K. Bailey-Dell, S. Zhou, K. E. Mercer, et al., The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme, J. Biol. Chem., 2004, 279, 24218–24225.
P. K. Selbo, A. Weyergang, M. S. Eng, M. Bostad, G. M. Maelandsmo, A. Hogset, et al., Strongly amphiphilic photosensitizers are not substrates of the cancer stem cell marker ABCG2 and provides specific and efficient light-triggered drug delivery of an EGFR-targeted cytotoxic drug, J. Controlled Release, 2012, 159, 197–203.
C. E. Olsen, K. Berg, P. K. Selbo, A. Weyergang, Circumvention of resistance to photodynamic therapy in doxorubicin-resistant sarcoma by photochemical internalization of gelonin, Free Radic. Biol. Med., 2013, 65, 1300–1309.
A. K. Larsen, A. E. Escargueil, A. Skladanowski, Resistance mechanisms associated with altered intracellular distribution of anticancer agents, Pharmacol. Ther., 2000, 85, 217–229.
N. Altan, Y. Chen, M. Schindler, S. M. Simon, Defective acidification in human breast tumor cells and implications for chemotherapy, J. Exp. Med., 1998, 187, 1583–1598.
P. J. Lou, P. S. Lai, M. J. Shieh, A. J. Macrobert, K. Berg, S. G. Bown, Reversal of doxorubicin resistance in breast cancer cells by photochemical internalization, Int. J. Cancer, 2006, 119, 2692–2698.
C. M. Lee, I. F. Tannock, Inhibition of endosomal sequestration of basic anticancer drugs: influence on cytotoxicity and tissue penetration, Br. J. Cancer, 2006, 94, 863–869.
R. A. Kramer, J. Zakher, G. Kim, Role of the glutathione redox cycle in acquired and de novo multidrug resistance, Science, 1988, 241, 694–697.
J. Chen, Reactive Oxygen Species and Drug Resistance in Cancer Chemotherapy, Austin J. Clin. Pathol., 2014, 1, 1017–1023.
M. Diehn, R. W. Cho, N. A. Lobo, T. Kalisky, M. J. Dorie, A. N. Kulp, et al., Association of reactive oxygen species levels and radioresistance in cancer stem cells, Nature, 2009, 458, 780–783.
G. H. Rodal, S. K. Rodal, J. Moan, K. Berg, Liposome-bound Zn(ii)-phthalocyanine. Mechanisms for cellular uptake and photosensitization, J. Photochem. Photobiol., B, 1998, 45, 150–159.
Y. N. Wang, H. Yamaguchi, J. M. Hsu, M. C. Hung, Nuclear trafficking of the epidermal growth factor receptor family membrane proteins, Oncogene, 2010, 29, 3997–4006.
D. J. Chen, C. S. Nirodi, The epidermal growth factor receptor: a role in repair of radiation-induced DNA damage, Clin. Cancer Res., 2007, 13, 6555–6560.
C. Li, M. Iida, E. F. Dunn, A. J. Ghia, D. L. Wheeler, Nuclear EGFR contributes to acquired resistance to cetuximab, Oncogene, 2009, 28, 3801–3813.
L. Prasmickaite, A. Hogset, P. K. Selbo, B. O. Engesaeter, M. Hellum, K. Berg, Photochemical disruption of endocytic vesicles before delivery of drugs: a new strategy for cancer therapy, Br. J. Cancer, 2002, 86, 652–657.
E. Verri, P. Guglielmini, M. Puntoni, L. Perdelli, A. Papadia, P. Lorenzi, et al., HER2/neu oncoprotein overexpression in epithelial ovarian cancer: evaluation of its prevalence and prognostic significance. Clinical study, Oncology, 2005, 68, 154–161.
B. Bull-Hansen, Y. Cao, K. Berg, E. Skarpen, M. G. Rosenblum, A. Weyergang, Photochemical activation of the recombinant HER2-targeted fusion toxin MH3-B1/rGel; Impact of HER2 expression on treatment outcome, J. Controlled Release, 2014, 182, 58–66.
R. Worthylake, L. K. Opresko, H. S. Wiley, ErbB-2 amplification inhibits down-regulation and induces constitutive activation of both ErbB-2 and epidermal growth factor receptors, J. Biol. Chem., 1999, 274, 8865–8874.
L. DeFazio-Eli, K. Strommen, T. Dao-Pick, G. Parry, L. Goodman, J. Winslow, Quantitative assays for the measurement of HER1-HER2 heterodimerization and phosphorylation in cell lines and breast tumors: applications for diagnostics and targeted drug mechanism of action, Breast Cancer Res., 2011, 13, R44.
K. Subik, J. F. Lee, L. Baxter, T. Strzepek, D. Costello, P. Crowley, et al., The Expression Patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by Immunohistochemical Analysis in Breast Cancer Cell Lines, Breast Cancer, 2010, 4, 35–41.
M. Bostad, K. Berg, A. Hogset, E. Skarpen, H. Stenmark, P. K. Selbo, Photochemical internalization (PCI) of immunotoxins targeting CD133 is specific and highly potent at femtomolar levels in cells with cancer stem cell properties, J. Controlled Release, 2013, 168, 317–326.
M. Bostad, M. Kausberg, A. Weyergang, C. E. Olsen, K. Berg, A. Hogset, et al., Light-Triggered, Efficient Cytosolic Release of IM7-Saporin Targeting the Putative Cancer Stem Cell Marker CD44 by Photochemical Internalization, Mol. Pharm., 2014, 11, 2764–2776.
A. Weyergang, L. H. Cheung, M. G. Rosenblum, K. A. Mohamedali, Q. Peng, J. Waltenberger, et al., Photochemical internalization augments tumor vascular cytotoxicity and specificity of VEGF121/rGel fusion toxin, J. Controlled Release, 2014, 180, 1–9.
C. H. Choi, ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal, Cancer Cell Int., 2005, 5, 30.
R. B. Wang, C. L. Kuo, L. L. Lien, E. J. Lien, Structure–activity relationship: analyses of p-glycoprotein substrates and inhibitors, J. Clin. Pharm. Ther., 2003, 28, 203–228.
C. Liu, G. Zhao, J. Liu, N. Ma, P. Chivukula, L. Perelman, et al., Novel biodegradable lipid nano complex for siRNA delivery significantly improving the chemosensitivity of human colon cancer stem cells to paclitaxel, J. Controlled Release, 2009, 140, 277–283.
H. Meng, M. Liong, T. Xia, Z. Li, Z. Ji, J. I. Zink, et al., Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and P-glycoprotein siRNA to overcome drug resistance in a cancer cell line, ACS Nano, 2010, 4, 4539–4550.
M. Leal, P. Sapra, S. A. Hurvitz, P. Senter, A. Wahl, M. Schutten, et al., Antibody-drug conjugates: an emerging modality for the treatment of cancer, Ann. N. Y. Acad. Sci., 2014, 1321, 41–54.
A. Illes, A. Jona, Z. Miltenyi, Brentuximab vedotin for treating Hodgkin’s lymphoma: an analysis of pharmacology and clinical efficacy, Expert Opin. Drug Metab. Toxicol., 2015, 11, 451–459.
D. J. Wong, S. A. Hurvitz, Recent advances in the development of anti-HER2 antibodies and antibody-drug conjugates, Ann. Transl. Med., 2014, 2, 122–5839.
E. L. Sievers, P. D. Senter, Antibody-drug conjugates in cancer therapy, Annu. Rev. Med., 2013, 64, 15–29.
F. Stirpe, S. Olsnes, A. Pihl, Gelonin, a new inhibitor of protein synthesis, nontoxic to intact cells. Isolation, characterization, and preparation of cytotoxic complexes with concanavalin A, J. Biol. Chem., 1980, 255, 6947–6953.
L. Barbieri, M. G. Battelli, F. Stirpe, Ribosome-inactivating proteins from plants, Biochim. Biophys. Acta, 1993, 1154, 237–282.
C. M. Pirie, B. J. Hackel, M. G. Rosenblum, K. D. Wittrup, Convergent potency of internalized gelonin immunotoxins across varied cell lines, antigens, and targeting moieties, J. Biol. Chem., 2011, 286, 4165–4172.
P. K. Selbo, M. Bostad, C. E. Olsen, V. T. Edwards, A. Høgset, A. Weyergang, K. Berg, Photochemical internalisation, a minimally invasive strategy for light-controlled endosomal escape of cancer stem cell-targeting therapeutics, Photochem. Photobiol. Sci., 2015
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weyergang, A., Berstad, M.E.B., Bull-Hansen, B. et al. Photochemical activation of drugs for the treatment of therapy-resistant cancers. Photochem Photobiol Sci 14, 1465–1475 (2015). https://doi.org/10.1039/c5pp00029g
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c5pp00029g