Abstract
Manganese (Mn2+) neurotoxicity from occupational exposure is well documented to result in a Parkinson-like syndrome. Although the understanding of Mn2+ cytotoxicity is still incomplete, both Mn2+ and Fe2+ can be transported via the divalent metal transporter 1 (DMT1), suggesting that competitive uptake might disrupt Fe2+ homeostasis. Here, we found that DMT1 overexpression significantly enhanced Mn2+ cytoplasmic accumulation and JNK phosphorylation, leading to a reduction in cell viability. Although a robust activation of autophagy was observed alongside these changes, it did not trigger autophagic cell death, but was instead shown to be essential for the degradation of ferritin, which normally sequesters labile Fe2+. Inhibition of ferritin degradation through the neutralization of lysosomal pH resulted in increased ferritin and enhanced cytoplasmic Fe2+ depletion. Similarly, direct Fe2+ chelation also resulted in aggravated Mn2+-mediated JNK phosphorylation, while Fe2+ repletion protected cells and this occurs via the ASK1-thioredoxin pathway. Taken together, our study presents the novel findings that Mn2+ cytotoxicity involves the depletion of the cytoplasmic Fe2+ pool and the increase in autophagy-lysosome activity is important to maintain Fe2+ homeostasis. Thus, Fe2+ supplementation could have potential applications in the prevention and treatment of Mn2+-mediated toxicity.
Similar content being viewed by others
Introduction
Although manganese (Mn2+) is a trace metal element vital for biological functions, chronic exposure to Mn2+ has been associated with the development of neurological dysfunction resembling Parkinson’s disease (PD)1. Airborne Mn2+ exposure in humans is a cause of neurotoxicity to the basal ganglia, resulting in a mixture of neuropsychiatric and motoric disturbances known as manganism2. Chronic Mn2+ exposure is associated with a greater risk of developing PD amongst miners and welders, as well as populations living near ferroalloy industries3. In addition, the use of methylcyclopentadienyl manganese tricarbonyl (MMT) as a gasoline additive in some parts of the world could pose a danger to public health as MMT has been shown to be toxic to dopaminergic neurons4. With its extensive use in various industries, Mn2+ exposure could be a silent pandemic affecting neuronal development, as well as the onset and course of neurodegeneration5. The proposed mechanism of Mn2+ uptake into the brain involves the divalent metal transporter 1 (DMT1), a 12-transmembrane domain protein found in a range of tissues including duodenum, kidney and brain and capable of transporting a number of divalent cations6. As both Mn2+ and Fe2+ rely heavily on DMT1 for cellular transport, prolonged exposure or chronic deficiency of either metal ion may interfere with the uptake and therefore the normal function of the other7. Indeed, Mn2+ levels in humans and animals have been observed to be influenced by Fe2+ status, specifically Mn2+ loading during Fe2+ deficiency7,8,9,10,11. While epidemiological and animal studies have demonstrated the detrimental effects of Mn2+exposure, the cell signaling pathways involved in Mn2+ toxicity and its interaction with Fe2+ are still not well understood. In our current study, we demonstrate that Mn2+ mediates cytotoxicity via depletion of cytoplasmic Fe2+ and activation of the JNK pathway. Importantly, we show that Fe2+-repletion suppressed Mn2+-mediated JNK activation, suggesting that Fe2+ supplementation can modify Mn2+-mediated cytotoxicity. Furthermore, our findings implicate the importance of the autophagic-lysosomal pathway in the degradation of ferritin since lysosomal inhibition further exacerbated cellular stress. Finally, we reduced JNK activation by overexpressing thioredoxin protein or inhibiting ASK1, suggesting the involvement of ASK1 upstream of the JNK pathway in Mn2+ toxicity. Taken together, our results provide evidence that Mn2+ cytotoxicity is related to Fe2+ depletion and the activation of JNK signaling via the thioredoxin/ASK1 pathway mediates cell death. These findings have important implications for the use of Fe2+ supplementation to reduce Mn2+ loading and toxicity in high risk populations.
Results
DMT1 overexpression in neuronal SH-SY5Y cells
To test the hypothesis that DMT1 mediates Mn2+ cytotoxicity, we used a previously generated geneticin-resistant stable SH-SY5Y cell line overexpressing DMT1 (S-DMT1)12 to transport Mn2+ into the cell. The expression of the DMT1-GFP fusion protein was verified using western blot analysis and fluorescence microscopy. DMT1-GFP protein is highly expressed in the S-DMT1 cells, as detected using DMT1B monoclonal antibody (Fig. 1A). The vector cell line overexpressing GFP protein (SGFP) showed the expression of the endogenous DMT1 at approximately 66 kDa while S-DMT1 cells showed further DMT1 immunoreactivity at 90 kDa and 250 kDa. The 90 kDa and 250 kDa bands correspond to DMT1-GFP and glycosylated DMT1-GFP fusion proteins13 respectively. Additionally, the DMT1-GFP protein was also viewed directly using fluorescence imaging to validate the cellular distribution of GFP compared to DMT1-GFP (Fig. 1B). In the S-DMT1 cells, DMT1-GFP was observed as puncta and located at the periphery of the cell, while SGFP cells showed diffuse cytoplasmic GFP expression. Indeed, studies of DMT1 transfected into non-neuronal cells such as Hep2 and CHO cells also confirm its plasma membrane and lysosomal localization14,15. Together, our data demonstrates that the localization of DMT1 overexpressed in SH-SY5Y cells is consistent with that of endogenous DMT1 in primary neuronal cells16.
DMT1-mediated Mn2+ uptake, cytoplasmic accumulation and reduction in cell viability
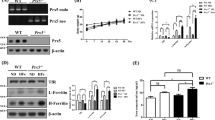
Previously, we have shown that the overexpression of DMT1 mediates robust cellular entry of Fe2+ into SH-SY5Y cells using the Fe55 uptake assay 12. As this assay measures the absolute accumulation of Fe2+, the difference between intracellular labile and non-labile Fe2+ could not be distinguished. To examine the accumulation of chelatable labile Fe2+ and Mn2+, cell-permeant Calcein-AM (a fluorescent dye whose intensity can be quenched by metal ions) was used. Calcein-AM is known to permeate the plasma membrane but does not readily enter subcellular organelles. This allows us to estimate the entry and cytoplasmic retention of labile Mn2+ and Fe2+ by measuring the amount of calcein fluorescence that is being quenched by the metal ions17. When S-DMT1 cells were treated with Mn2+ for 30 min, there was a concentration-dependent quenching of calcein fluorescence (Fig. 2A). In contrast, while Fe2+ treatment mediated a reduction in calcein fluorescence, the reduction itself was not concentration-dependent (Fig. 2B). This indicates that DMT1 mediates robust entry and accumulation of labile Mn2+. In parallel with this accumulation, S-DMT1 cells treated with Mn2+ for 24 h displayed significant reduction in cell viability compared to vector SGFP cells (Fig. 2C). Unlike Mn2+ however, increasing concentrations of Fe2+ were not associated with the progressive accumulation of its labile form. Moreover, in contrast to Mn2+, Fe2+ treatment did not result in any significant reduction in S-DMT1 cell viability compared to SGFP cells (Fig. 2D). This suggests that cells may have a protective mechanism against the build-up of labile iron. A likely candidate in this case would be the ferritin heavy chain (FTH1), which converts Fe2+ into the non-labile Fe3+ form. Indeed, we found that the level of ferritin was dramatically upregulated in cells treated with Fe2+ for 12 h and this occurred in a concentration-dependent fashion (Fig. 2E).
Uptake and cytoplasmic accumulation of Mn2+.
Calcein quenching assay was performed on S-DMT1 cells treated with either Mn2+ or Fe2+ for 30 min. The effect of cytoplasmic accumulation on cell viability was determined using MTT assay. (a) Mn2+ treatment reduced calcein fluorescence in a concentration-dependent manner (compared to untreated, **p < 0.01; ***p < 0.001, n = 4, one-way ANOVA, Dunnett post hoc test). (b) Fe2+ treatment reduced calcein fluorescence (compared to untreated, *p < 0.05, **p < 0.01, ***p < 0.001, n = 4) but not in a concentration-dependent manner. (c) MTT assay of Mn2+ treatment of S-DMT1 cells for 24 h reduced cell viability compared to vector cells, especially for 0.5 and 1 mM Mn2+ (*p < 0.05, **p < 0.01, n = 4, two-way ANOVA, Bonferroni post hoc test). (d) MTT assay of Fe2+ treatment of S-DMT1 cells for 24 h did not show any significant reduction in cell viability compared to vector cells (n = 4). (e) Representative western blot showing upregulation of ferritin heavy chain (FTH1) protein in a Fe2+ concentration-dependent fashion in S-DMT1 cells treated for 12 h. Corresponding bar chart displays ferritin protein levels with Fe2+ treatment (**p < 0.01, ***p < 0.001, n = 3), with error bars representing standard error of mean (S.E.M.). β-actin was used as a loading control.
Cell viability reduction mediated by Mn2+ is associated with the activation of MAP Kinase JNK pathway
Given that the MAP kinase stress-induced pathway plays an important role in the regulation of cell proliferation, differentiation, survival and apoptosis18, we next sought to examine the potential effect of Mn2+ on this pathway. At 1 mM Mn2+, S-DMT1 cells showed a dramatic increase in JNK phosphorylation in a time-dependent fashion compared to SGFP cells (Fig. 3A, blots were developed with the same exposure time). Treatment with 1 mM Fe2+ did not significantly increase JNK phosphorylation in both SGFP and S-DMT1 cells. Since Mn2+ treatment of S-DMT1 cells resulted in enhanced JNK phosphorylation, we next questioned whether JNK phosphorylation was responsible for Mn2+-mediated reduction in cell viability. To examine this, we used 25 μM of SP600125, an inhibitor of JNK phosphorylation. S-DMT1 cells treated with 0.5 or 1 mM Mn2+ for 12 h showed increased JNK phosphorylation compared to untreated cells. This phosphorylation was significantly suppressed with addition of SP600125 (Fig. 3B). Notably, the more than 2-fold increase in JNK phosphorylation mediated by 1 mM Mn2+ treatment was totally abolished in the presence of SP600125. Under phase contrast microscopy, S-DMT1 cells treated with SP600125 showed reduced vulnerability to Mn2+ compared to cells treated with Mn2+ alone. Indeed, using the MTT cell viability assay, S-DMT1 cells treated with a range of Mn2+ concentrations for 24 h were significantly protected against Mn2+ cytotoxicity in the presence of SP600125 (Fig. 3C). The data suggests that JNK activation plays an important role in Mn2+-mediated cell death.
Mn2+ cytotoxicity is associated with JNK activation.
(a) Representative western blot showing time-dependent JNK phosphorylation upon treatment with 1 mM of Mn2+ in both S-DMT1 and vector cells. S-DMT1 cells displayed more robust JNK phosphorylation compared to vector cells. 1 mM Fe2+ treatment of both cell lines did not increase JNK phosphorylation. (b) S-DMT1 cells treated with 1 mM of Mn2+ for 12 h increased (*p < 0.05) JNK phosphorylation compared to untreated. This JNK phosphorylation induced by Mn2+ was significantly (^p < 0.05) reduced with SP600125 (25 μM) treatment. Bar chart shows fold change of phosphorylated JNK protein levels over untreated (n = 3, Student’s t-test), with error bars representing standard error of mean (S.E.M.). Corresponding full-length blots are presented in Supplementary Fig. 1. (c) MTT cell viability assay showing the protective effect of SP600125 against Mn2+-mediated cytotoxicity (*p < 0.05, ***p < 0.001, n = 3, two-way ANOVA, Bonferroni post hoc test). Phase contrast images show the reduced vulnerability of S-DMT1 cells to 0.5 mM Mn2+ cytotoxicity when treated with SP600125 for 24 h.
Autophagic activity associated with Mn2+ treatment is protective during Mn2+ stress
In our previous work, we showed that chronic Fe2+ overload together with α-synuclein A53T overexpression led to an increase in autophagic activity and cell death12. We thus investigated whether autophagy could be involved in Mn2+-mediated cytotoxicity. Upon autophagy induction, cytoplasmic LC3-I is lipidated to LC3-II, which then localizes to autophagic vesicles19. To determine the level of autophagic activity in Mn2+-treated cells, naïve SH-SY5Y cells were transfected with the mRFP-GFP tandem fluorescence-tagged LC3 construct (tf-LC3) for 24 h. Transfected cells treated with 0.5 mM Mn2+ for 6 h showed induction of autophagy as indicated by the increase in average red LC3 puncta per cell compared to untreated cells (Fig. 4A). The green GFP puncta associated with tf-LC3 was lost in Mn2+-treated cells presumably due to the quenching of the GFP signal within the acidic lysosomes. To reverse the loss of GFP puncta signal, cells were co-treated with Mn2+ and NH4Cl (which neutralizes the pH of lysosomes) and the GFP signal reappeared and colocalized with the red LC3 puncta in the merged image. Cells treated with either 0.5 mM Fe2+ or 0.5 mM Fe2+ + Mn2+ for 6 h did not show any significant increase in the number of LC3 puncta, suggesting that autophagy was not significantly changed with these treatments. Cells treated with EBSS for 6 h (which promotes starvation-induced autophagy) was used as a positive control for autophagy induction. In addition, we used western blot analysis to detect the level of LC3-II, which acts as a marker for autophagy. S-DMT1 cells treated with 0.5 mM Mn2+ for 12 h showed a substantial accumulation of LC3-II compared to untreated cells (Supplementary Fig. 2A), while 0.5 mM Fe2+ and 0.5 mM Fe2+ + Mn2+ treatments did not result in any significant change. The data suggests that Mn2+ mediates robust autophagy activation in S-DMT1 cells, while this was not observed with Fe2+. Importantly, by simply co-treating with Fe2+, the activation of autophagy induced by Mn2+ could be reversed.
Mn2+-mediates an increase in autophagy, which is protective during Mn2+ stress.
(a) SH-SY5Y cells were transfected with tandem fluorescence LC3 containing GFP and RFP for 24 h. Cells treated with 0.5 mM Mn2+ for 6 h had a significantly increased (***p < 0.001) number of red LC3 puncta per cell (at least 50 cells per experiment). 0.5 mM Fe2+ treatment did not show any significant increase in LC3 puncta and co-treatment of 0.5 mM Fe2+ and 0.5 mM Mn2+ substantially (***p < 0.001) reduced LC3 puncta compared to Mn2+ alone. Treatment with NH4Cl neutralized lysosome pH, allowing the appearance of yellow LC3 puncta indicating autophagic flux. EBSS was used as a positive control for autophagy induction. Bar chart shows the quantification of average LC3 red puncta per cell for various treatments (n = 3, Student’s t-test). (b) Representative western blot of ATG5+/+ and ATG5−/− MEF with or without NH4Cl treatment. NH4Cl inhibited autophagy degradation, allowing the accumulation and visualization of the LC3-II protein in normal ATG5+/+ MEF but not ATG5−/−. (c) MTT assay of ATG5+/+ and ATG5−/− MEF treated with Mn2+ for 24 h showing the significant reduction (*p < 0.05, n = 3, two-way ANOVA, Bonferroni post hoc test) in ATG5−/− cell viability at 2 mM Mn2+ compared to ATG5+/+ MEF. Corresponding phase contrast images of 2 mM Mn2+ treatment for 24 h showed similar increased vulnerability of ATG5−/− MEF.
With the robust activation of autophagy upon Mn2+ stress, we examined the effect of autophagy on cell viability by using ATG5 autophagy-deficient mouse embryonic fibroblast cells (ATG5−/− MEF). ATG5 is an important gene required for the initiation of autophagy20,21. Treatment of ATG5+/+ and ATG5−/− MEF with NH4Cl for 12 h neutralized lysosome pH, preventing autophagic degradation and allowing the accumulation of LC3-II protein in the ATG5+/+ MEF but not in ATG5−/− (Fig. 4B). This verifies the failure of ATG5−/− MEF to initiate autophagy. We then carried out the MTT cell viability assay and found that ATG5−/− MEF treated with 2 mM Mn2+ showed significant reduction in cell viability compared to ATG5+/+ MEF. Under phase contrast microscopy, autophagy deficient ATG5−/− MEF also showed increased vulnerability to Mn2+ cytotoxicity (Fig. 4C). As lysosomal acidification is important for proteolysis of ferritin to extract labile Fe2+ 22, the inhibition of lysosomal function by NH4Cl could potentially aggravate Mn2+-mediated cytotoxicity. To test this, we treated S-DMT1 cells with NH4Cl and found that Mn2+-mediated JNK phosphorylation was indeed significantly higher from 2 h onwards compared to Mn2+ alone (Supplementary Fig. 2B). In addition the inhibition of lysosomal function could be seen from the accumulation of autophagic LC3-II, which would otherwise be degraded by the lysosomes.
Mn2+ induces ferritin degradation and Fe2+ chelation exacerbates Mn2+-mediated JNK activation
Since addition of Fe2+ appeared to reduce the level of autophagy induced by Mn2+ (Fig. 4A), we next investigated the role of Mn2+-mediated autophagy with regards to Fe2+ homeostasis. We found that S-DMT1 cells treated with 0.5 or 1 mM Mn2+ for 24 h showed a significant reduction in ferritin protein compared to untreated (Fig. 5A). In contrast, treatment with 0.5 or 1 mM Fe2+ resulted in a concentration-dependent upregulation of ferritin. Importantly, co-treatment of 0.5 mM Mn2+ and 0.5 mM Fe2+ prevented the complete loss of ferritin compared to 0.5 mM Mn2+ treatment alone. This led us to hypothesize that Mn2+ treatment resulted in enhanced degradation of ferritin due to cellular Fe2+ depletion. We then proceeded to determine if autophagy was involved in Mn2+-mediated ferritin loss. S-DMT1 cells were first exposed to Fe2+ for 12 h or left untreated. Subsequently, this was changed to normal media or 0.5 mM Mn2+ for another 12 h. Ferritin protein was dramatically reduced on treatment with Mn2+ (Fig. 5B). However, the addition of autophagy inhibitor NH4Cl almost completely prevented Mn2+-mediated degradation of ferritin.
Mn2+ induces ferritin degradation and Fe2+ chelation exacerbates Mn2+-mediated JNK activation.
(a) Representative western analysis of ferritin levels upon Fe2+ and Mn2+ treatment. 0.5 and 1 mM of Fe2+ for 24 h resulted in a concentration-dependent increase in ferritin levels in S-DMT1 cells. In contrast, both 0.5 and 1 mM Mn2+ reduced ferritin levels compared to untreated. Ferritin loss associated with 0.5 mM Mn2+ was partially reversed when co-treated with 0.5 mM Fe2+. (b) Representative western analysis of S-DMT1 cells pretreated with 0.5 mM Fe2+ for 12 h (to upregulate ferritin) before treatment with 0.5 mM Mn2+ for another 12 h, with or without autophagy inhibition. In Fe2+ pretreated cells, incubation with Mn2+ significantly (**p < 0.01) reduced ferritin levels compared to Fe2+ treatment alone. With NH4Cl, Mn2+-induced reduction of ferritin is significantly suppressed (*p < 0.05) compared to Mn2+ without inhibitor. Corresponding bar chart shows ferritin protein fold change over Fe2+ treatment (n = 3, Student’s t-test), with error bars representing standard error of mean (S.E.M.). (c) Increasing concentration of Fe2+ led to marked ferritin accumulation in autophagy deficient ATG5−/− MEF compared to ATG5+/+. Autophagy deficiency in the ATG5−/− MEF was verified through the increase in mitochondrial SOD2 compared to ATG5+/+ MEF. (d) Mn2+ treatment together with Fe2+ chelation by 1,10-phenanthroline for 12 h resulted in increased JNK phosphorylation compared to Mn2+ alone (**p < 0.01, n = 4, Student’s t-test). In contrast, phenanthroline in the presence of Fe2+ did not result in increased JNK phosphorylation due to sufficient Fe2+. Similarly, addition of Fe2+ to Mn2+/phenanthroline reduced JNK phosphorylation (*p < 0.05).
Since lysosomal acidification is essential for the degradation of ferritin in order to release Fe2+ 23, we validated the importance of the basal autophagy-lysosomal pathway for the maintenance of Fe2+ homeostasis. Immunoblotting of ATG5−/− MEF treated with increasing concentrations of Fe2+ for 12 h showed dramatic upregulation and accumulation of ferritin protein compared to ATG5+/+ MEF (Fig. 5C). In addition, mitochondrial SOD2 was observed to be relatively higher (independent of Fe2+ treatment) in the ATG5−/− compared to ATG5+/+ MEF, indicating a deficiency in autophagic clearance of mitochondria these cells. This indicates that basal autophagy is indeed important to control ferritin degradation in response to changes in cellular Fe2+.
As Mn2+ treatment interferes with Fe2+ homeostasis by increasing autophagic clearance of ferritin, we asked whether enhancement of iron stores via Fe2+ repletion could abrogate Mn2+-associated JNK phosphorylation. S-DMT1 cells pre-exposed to 0.5 mM Fe2+ for 12 h showed increase in ferritin protein, while JNK phosphorylation remained unchanged (Supplementary Fig. 3A). In contrast, Mn2+ treatment increased JNK phosphorylation and reduced ferritin level. Finally, Fe2+-repletion of Mn2+ treated cells reduced JNK phosphorylation and increased ferritin protein compared to Mn2+ treatment alone. Since treatment with Fe2+ could reduce Mn2+-mediated JNK phosphorylation, we next examined the viability of S-DMT1 cells using the MTT assay. Indeed, cells treated with both Mn2+ and Fe2+ were significantly protected against Mn2+ cytotoxicity (Supplementary Fig. 3B). Additionally, the cells appeared healthier as observed using phase contrast microscopy.
We next examined the response of S-DMT1 cells to the reverse experiment by inducing Fe2+ deficiency with the cell permeable Fe2+ chelator 1,10-phenanthroline. In contrast to Fe2+ repletion, Fe2+ depletion resulted in significant toxicity. Treatment with 75 μM phenanthroline (Phen) alone for 12 h led to JNK phosphorylation, which could be reversed by supplementation with 0.5 mM Fe2+ (Fig. 5D). More importantly, Phen treatment exacerbated Mn2+ toxicity, resulting in a 2.6-fold increase in JNK phosphorylation. This, in turn, could be reduced by co-treatment with Fe2+, leading to a reduction of JNK phosphorylation for Fe2+/Mn2+/Phen compared to Mn2+/Phen. The results demonstrate the toxic effect of Fe2+ depletion and that enhancement of iron stores as well as extracellular competition between Fe2+ and Mn2+ for entry into cell via DMT1 could rescue Mn2+-mediated cytotoxicity.
Thioredoxin overexpression and ASK1 inhibition reverse JNK phosphorylation mediated by Mn2+ and lysosomal inhibition
Cellular Fe2+ depletion using Fe2+ chelators has been shown to stimulate the JNK pathway via the dissociation of the ASK1-thioredoxin complex, activation of ASK1 and the subsequent phosphorylation of JNK24. Hence, we sought to clarify the link between Mn2+-mediated JNK phosphorylation and cellular Fe2+ depletion by assessing the level of phosphorylated JNK in the presence of thioredoxin overexpression. S-DMT1 cells transfected with thioredoxin for 24 h showed a suppression of Mn2+-mediated JNK phosphorylation compared to vector-transfected cells. Additionally, thioredoxin overexpression reduced JNK phosphorylation induced by Mn2+ and NH4Cl (Fig. 6A). We then investigated whether thioredoxin overexpression could rescue cells from Mn2+ cytotoxicity. Mn2+ treatment of S-DMT1 cells transfected with thioredoxin for 24 h showed a significant increase in cell viability compared to Mn2+ treatment alone (Fig. 6B). Thioredoxin overexpression also increased cell viability for Mn2+ and NH4Cl treatment. Finally, we used NQDI-1, an ASK1 inhibitor25, to investigate the involvement of ASK1 and the downstream components MKK4 and JNK26. On treatment with Mn2+, MKK4 and JNK showed robust activation and this was reduced when NQDI-1 was added to inhibit ASK1 activity (Fig. 6C). Co-treatment of Mn2+ with Fe2+ also reduced MKK4 and JNK activation, presumably because Fe2+ supplementation reduced ASK1-thioredoxin dissociation and subsequent ASK1 activation. Taken together, this suggests that Mn2+ exposure results in cellular Fe2+ disruption with a concomitant increase in JNK phosphorylation via the thioredoxin/ASK1/MKK4 pathway (Fig. 7).
Thioredoxin overexpression and ASK1 inhibition reduce Mn2+-mediated JNK phosphorylation via the thioredoxin/ASK1/MKK4 pathway.
(a) Representative western blot and bar chart showing the reduction in JNK phosphorylation mediated by thioredoxin transfection of Mn2+ and NH4Cl treated S-DMT1 cells (compared to vector transfected cells, *p < 0.05, **p < 0.01, n = 4, Student’s t-test). Error bars represent standard error of mean (S.E.M.). (b) MTT assay of thioredoxin transfected S-DMT1 cells treated for 24 h with Mn2+ or Mn2+ with NH4Cl (*p < 0.05, compared to corresponding vector transfected cells, n = 4). (c) Representative western blot and corresponding bar chart showing the reduction in MKK4 and JNK phosphorylation mediated by 12 h treatment with 10 μM ASK1 inhibitor NQDI-1 in S-DMT1 cells (*p < 0.05, **p < 0.01, ***p < 0.001, n = 4, Student’s t-test). β-actin was used as a control for equal loading.
A proposed model of cellular Mn2+ toxicity via DMT1.
(a) Under normal conditions, cells acquire adequate amounts of Fe2+ through cellular uptake via DMT1 and degradation of ferritin to maintain the labile iron pool (LIP). (b) However, Mn2+ overexposure competes with Fe2+ for uptake by DMT1. In addition, the accumulation Mn2+ in the cytoplasm disrupts LIP, resulting in enhanced autophagy to degrade ferritin for Fe2+ mobilization. Mn2+-mediated cellular iron depletion also results in the activation of JNK via the TRX-ASK1 pathway, leading to cell death.
Discussion
Nutritional iron deficiency affects a significant proportion of the world population. In 2011, it was estimated that around 800 million women and children suffer from anemia, of which 50% is attributed to iron-deficiency27. An important consequence of iron deficiency is an apparent heightened risk of heavy-metal poisoning, especially in children, which may affect their neurodevelopment. Iron-deficient individuals have an increased absorption capacity that is not specific to iron, resulting in increased absorption of other divalent heavy metals such as manganese, lead and cadmium28. Prevention of iron deficiency could thus reduce the number of children susceptible to heavy-metal poisoning from contaminated water, lead paint and pollution from automobile, industrial or welding fumes27.
Since the DMT1 transporter has been shown to mediate the uptake of both Mn2+ and Fe2+ in the brain29, we generated SH-SY5Y stable cell lines overexpressing DMT1 to better understand the signaling pathways involved in the interaction of these two ions. DMT1 facilitated a concentration-dependent uptake of Mn2+ into the cytoplasm. This cytoplasmic accumulation of labile Mn2+ contributed substantially to cell toxicity since there is no known storage protein for Mn2+. In contrast, cells treated with Fe2+ only showed a 25% increase in labile Fe2+, which did not significantly affect cell viability. This is due to the presence and upregulation of ferritin protein upon Fe2+ exposure. Ferritin is a Fe3+-storage protein with ferroxidase activity that protects cells against Fe2+-mediated cytotoxicity30.
Although Mn2+ and Fe2+ share similar physical and chemical properties, the cellular requirement for each metal ion is highly specific in order for them to catalyze reactions in their respective metabolic pathways. Consequently, in the event of cellular Mn2+ overexposure, the accumulation of this ion may interfere with the metabolic processes of Fe2+-requiring enzymes. With uptake by DMT1, the level of Mn2+ might reach a concentration high enough to interfere with the synthesis of Fe2+-requiring enzymes or even directly displace Fe2+. In our study, pre-treatment as well as co-treatment with Fe2+ rescued cells from Mn2+-mediated cytotoxicity. It is possible that co-treatment resulted in Fe2+ competing with Mn2+ for uptake via DMT1, reducing Mn2+ entry into the cell, while pre-treatment increased stores in ferritin that allowed subsequent Fe2+ release to counter the toxic effects of Mn2+. Thus, there appears to be a delicate balance of cellular Fe2+ which could be disrupted by Mn2+ overexposure (Fig. 7).
We also found that autophagy was essential for the degradation of iron-laden ferritin in order to maintain the necessary level of cellular Fe2+ during Mn2+ exposure. This was corroborated through the inhibition of lysosome function which prevented the degradation of ferritin and hence failure of Fe2+ release. Indeed, the role of autophagy in maintaining cellular Fe2+ balance is supported by the recent identification of the nuclear receptor coactivator 4 (NCOA4), which is responsible for the selective targeting of ferritin to the lysosomes for protein turnover31. NCOA4 co-localizes with ferritin and LC3B in autophagosomes upon upregulation of ferritin by ferric ammonium citrate. In addition, either ATG5 or NCOA4 knockdown through RNA interference retarded ferritin turnover. This provided a molecular explanation on how the bioavailability of Fe2+ is maintained within the cell through autophagy, a process which the authors referred to as ‘ferritinophagy’.
In summary, we elucidate for the first time the cellular mechanisms of how Mn2+ cytotoxicity is closely linked to the disruption of Fe2+ homeostasis. Mn2+ interferes with Fe2+ balance via entry through DMT1 as well as disruption to intracellular signaling mechanisms. Specifically, we found that autophagy is upregulated upon Mn2+ treatment and this does not lead to autophagic cell death, but instead plays a pro-survival role. Autophagy is paramount during Mn2+ stress in order to degrade ferritin, release labile Fe2+ and restore Fe2+ balance. Consequently, inhibition of the autophagy-lysosomal pathway and subsequent failure to degrade ferritin aggravated Mn2+ cellular stress. We also demonstrated that JNK signaling during Mn2+ cytotoxicity could be reduced with Fe2+ repletion, supporting the finding that Fe2+ depletion is involved during Mn2+ stress. These findings could be extended with further studies to determine the potential of Fe2+ supplementation to counter Mn2+ toxicity in populations at risk of chronic Mn2+ exposure.
Methods
Generation of DMT1-GFP and Vector Stable Cell Lines
cDNA of DMT1 (Accession: AF153279) without the iron-response element (IRE) at the 3′ UTR was subcloned into a GFP vector to generate DMT1-GFP. The generation and characterization of the DMT1 stable cell lines were performed by our laboratory and described previously12. The DMT1 stable cell line (S-DMT1) and empty vector control (SGFP) were maintained in 200 μg/ml G418 (Gibco) supplemented DMEM containing 10% FBS and 1% Pen/Strep in 5% CO2 at 37 °C. The TRX1-Flag plasmid was obtained from Addgene (plasmid: 21283) and was previously described32.
ATG5−/− Mouse Embryonic Fibroblast (MEF)
The autophagy-deficient immortalized MEF cells were kind gifts from Dr. Noboru Mizushima from Tokyo Medical and Dental University, via Dr Shen Han Ming, Department of Physiology, NUS. The MEF cells were maintained in DMEM containing 10% FBS and 1% Pen/Strep in 5% CO2 at 37 °C.
Materials
Reagents were from Sigma Aldrich unless otherwise stated. NH4Cl (30 mM) and 1,10-Phenanthroline (75 μM) were dissolved in distilled water. SP600125 (25 μM) and NQDI-1 (10 μM, Cayman Chemical) were dissolved in DMSO. Mn2+ was purchased as a 1 M MgCl2 solution, while Fe2+ was prepared fresh by dissolving FeCl2 in 0.1 M HCl. Amino acid-free Earl’s balanced salt solution (EBSS) was used to induce autophagy via complete starvation.
Cell transfection and treatment
Cell transfection was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. Cells of at least 70% confluency were transfected with the desired plasmid constructs for 24 h before being subjected to various treatments.
Overexpression of LC3
The mRFP-GFP tandem fluorescent-tagged LC3 (tf-LC3) construct was previously described in our work12. Briefly, mRFP retained its red fluorescence in acidic lysosomes, but GFP fluorescence was quenched and could be recovered by neutralizing lysosome pH with NH4Cl. Transfection of tf-LC3 into SH-SY5Y cells was performed using Lipofectamine 2000 (Invitrogen). After 24 h, the cells were treated with various metal ions and chemical inhibitors for 6 h before fixing with 2.5% PFA and viewing with a confocal microscope (Olympus).
Calcein-AM Metal Quenching Assay
Calcein acetoxymethylester (Calcein-AM, 0.25 μM) was dissolved in DMSO. Briefly, cells were treated with metal ions for 30 min, then rinsed with PBS and incubated with calcein-AM in DMEM for 20 min at 37 °C, 5% CO2. The cells were rinsed in PBS followed by complete lysis in RIPA buffer containing 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS and 50 mM Tris, pH 8. The supernatant was transferred to a 96-well plate for fluorescence analysis at 488/518 nm wavelength (Tecan Infinite 200 PRO multimode reader). The fluorescence of the lysates was normalized to protein concentration determined using BCA method (Thermo Scientific).
Cell viability assay
Cell viability was determined using the MTT Cell Proliferation Kit (Roche) according to the manufacturer’s protocol. Cells were seeded into a 96-well plate to reach a confluency of 70%. The cells were then treated with Mn2+, Fe2+ or other chemicals for 24 h before analysis using MTT.
Protein Lysis and Western Blot Analysis
Protein extraction was carried out by lysing cells in PBS buffer containing 1% Triton X-100 with added protease and phosphatase inhibitors (Roche). Protein content was determined using Bradford assay (Pierce). Approximately 25 to 70 μg of total protein was resolved on 8, 10 or 12% SDS-PAGE gel before transferring onto PVDF membrane (Millipore) for detection using ECL reagent (Pierce). The following antibodies were used: anti-phospho-JNK, anti-JNK, anti-phospho-MKK4, anti-MKK4, anti-FTH1 (Cell Signaling Technology), anti-TRX (Santa Cruz), anti-LC3B (Abgent), anti-SOD2 (Abcam), anti-actin and HRP-conjugated anti-rabbit and anti-mouse (Sigma).
Densitometric Analysis and Statistics
Western blot band intensities were analyzed using the densitometric analysis software Quantity One (Biorad). The intensities of the specified bands were normalized to β-actin. Data was expressed as standard error of mean (S.E.M) of at least three independent experiments. Experimental data was compared using Student’s t-test, one-way ANOVA with Dunnett post hoc test or two-way ANOVA with Bonferroni post hoc test and was considered statistically significant when p < 0.05.
Additional Information
How to cite this article: Tai, Y. K. et al. Iron mitigates DMT1-mediated manganese cytotoxicity via the ASK1-JNK signaling axis: Implications of iron supplementation for manganese toxicity. Sci. Rep. 6, 21113; doi: 10.1038/srep21113 (2016).
References
Dobson, A. W., Erikson, K. M. & Aschner, M. Manganese neurotoxicity. Ann NY Acad Sci 1012, 115–128 (2004).
Aschner, M., Erikson, K. M. & Dorman, D. C. Manganese dosimetry: species differences and implications for neurotoxicity. Crit Rev Toxicol 35, 1–32 (2005).
Weiss, B. Economic implications of manganese neurotoxicity. Neurotoxicology 27, 362–368 (2006).
Kitazawa, M., Wagner, J. R., Kirby, M. L., Anantharam, V. & Kanthasamy, A. G. Oxidative stress and mitochondrial-mediated apoptosis in dopaminergic cells exposed to methylcyclopentadienyl manganese tricarbonyl. The Journal of pharmacology and experimental therapeutics 302, 26–35 (2002).
Grandjean, P. & Landrigan, P. J. Neurobehavioural effects of developmental toxicity. The Lancet. Neurology 13, 330–338 (2014).
Gunshin, H. et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388, 482–488 (1997).
Erikson, K. M., Syversen, T., Aschner, J. L. & Aschner, M. Interactions between excessive manganese exposures and dietary iron-deficiency in neurodegeneration. Environ Toxicol Pharmacol 19, 415–421 (2005).
Kim, J. et al. Iron-responsive olfactory uptake of manganese improves motor function deficits associated with iron deficiency. PloS one 7, e33533 (2012).
Chandra, S. V. & Shukla, G. S. Role of iron deficiency in inducing susceptibility to manganese toxicity. Archives of toxicology 35, 319–323 (1976).
Fitsanakis, V. A. et al. A chronic iron-deficient/high-manganese diet in rodents results in increased brain oxidative stress and behavioral deficits in the morris water maze. Neurotox Res 15, 167–178 (2009).
Erikson, K. M., Shihabi, Z. K., Aschner, J. L. & Aschner, M. Manganese accumulates in iron-deficient rat brain regions in a heterogeneous fashion and is associated with neurochemical alterations. Biological trace element research 87, 143–156 (2002).
Chew, K. C. et al. Enhanced autophagy from chronic toxicity of iron and mutant A53T alpha-synuclein: implications for neuronal cell death in Parkinson disease. J Biol Chem 286, 33380–33389 (2011).
Tabuchi, M., Tanaka, N., Nishida-Kitayama, J., Ohno, H. & Kishi, F. Alternative splicing regulates the subcellular localization of divalent metal transporter 1 isoforms. Molecular biology of the cell 13, 4371–4387 (2002).
Tabuchi, M., Yoshimori, T., Yamaguchi, K., Yoshida, T. & Kishi, F. Human NRAMP2/DMT1, which mediates iron transport across endosomal membranes, is localized to late endosomes and lysosomes in HEp-2 cells. J Biol Chem 275, 22220–22228 (2000).
Picard, V., Govoni, G., Jabado, N. & Gros, P. Nramp 2 (DCT1/DMT1) expressed at the plasma membrane transports iron and other divalent cations into a calcein-accessible cytoplasmic pool. J Biol Chem 275, 35738–35745 (2000).
Pelizzoni, I., Zacchetti, D., Smith, C. P., Grohovaz, F. & Codazzi, F. Expression of divalent metal transporter 1 in primary hippocampal neurons: reconsidering its role in non-transferrin-bound iron influx. J Neurochem 120, 269–278 (2012).
Tenopoulou, M., Kurz, T., Doulias, P. T., Galaris, D. & Brunk, U. T. Does the calcein-AM method assay the total cellular ‘labile iron pool’ or only a fraction of it? Biochem J 403, 261–266 (2007).
Kim, E. K. & Choi, E. J. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta 1802, 396–405 (2010).
Gurusamy, N. & Das, D. K. Detection of cell death by autophagy. Methods Mol Biol 559, 95–103 (2009).
Yu, L. et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science 304, 1500–1502 (2004).
Byun, J. Y. et al. The Rac1/MKK7/JNK pathway signals upregulation of Atg5 and subsequent autophagic cell death in response to oncogenic Ras. Carcinogenesis 30, 1880–1888 (2009).
Zhang, Y. et al. Lysosomal proteolysis is the primary degradation pathway for cytosolic ferritin and cytosolic ferritin degradation is necessary for iron exit. Antioxid Redox Signal 13, 999–1009 (2010).
Asano, T. et al. Distinct mechanisms of ferritin delivery to lysosomes in iron-depleted and iron-replete cells. Mol Cell Biol 31, 2040–2052 (2011).
Yu, Y. & Richardson, D. R. Cellular Iron Depletion Stimulates the JNK and p38 MAPK Signaling Transduction Pathways, Dissociation of ASK1-Thioredoxin and Activation of ASK1. J Biol Chem 286, 15413–15427 (2011).
Volynets, G. P. et al. Identification of 3H-Naphtho[1,2,3-de]quinoline-2,7-diones as Inhibitors of Apoptosis Signal-Regulating Kinase 1 (ASK1). J Med Chem 54, 2680–2686 (2011).
Ichijo, H. et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275, 90–94 (1997).
WHO. The global prevalence of anaemia in 2011 (World Health Organization, Geneva, 2015).
WHO. Iron deficiency anaemia: assessment, prevention and control. A guide for programme managers. doi: WHO/NHD/01.3 (2001) Available at: http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/WHO_NHD_01.3/en/. (Accessed: 16 August 2015).
Au, C., Benedetto, A. & Aschner, M. Manganese transport in eukaryotes: the role of DMT1. Neurotoxicology 29, 569–576 (2008).
Berberat, P. O. et al. Heavy chain ferritin acts as an antiapoptotic gene that protects livers from ischemia reperfusion injury. FASEB J 17, 1724–1726 (2003).
Mancias, J. D., Wang, X., Gygi, S. P., Harper, J. W. & Kimmelman, A. C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 509, 105–109 (2014).
Liu, Y. & Min, W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ Res 90, 1259–1266 (2002).
Acknowledgements
This work is supported by Biomedical Research Council (BMRC) Grant R-185-000-093-305 (to T.W.S.) and National Medical Research Council (NMRC) Grant NMRC-EDG 10nov065 (to K.L.L.). We thank Dr. Noboru Mizushima from Tokyo Medical and Dental University for providing the ATG5−/− mouse embryonic fibroblast cells via Dr. Shen Han Ming, Department of Physiology, NUS.
Author information
Authors and Affiliations
Contributions
Y.K.T., K.L.L. and T.W.S. designed and interpreted the experiments. Y.K.T. and K.C.C. did the bulk of the experiments, while B.W.T. did additional experiments. Y.K.T. and K.C.C. generated the figures, while Y.K.T. and T.W.S. wrote the manuscript with advice from K.L.L.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Tai, Y., Chew, K., Tan, B. et al. Iron mitigates DMT1-mediated manganese cytotoxicity via the ASK1-JNK signaling axis: Implications of iron supplementation for manganese toxicity. Sci Rep 6, 21113 (2016). https://doi.org/10.1038/srep21113
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep21113
This article is cited by
-
Alteration of iron (Fe), copper (Cu), zinc (Zn), and manganese (Mn) tissue levels and speciation in rats with desferioxamine-induced iron deficiency
BioMetals (2021)
-
Manganese Acts upon Insulin/IGF Receptors to Phosphorylate AKT and Increase Glucose Uptake in Huntington’s Disease Cells
Molecular Neurobiology (2020)
-
Affected energy metabolism under manganese stress governs cellular toxicity
Scientific Reports (2017)
-
Transgenic Mice Overexpressing the Divalent Metal Transporter 1 Exhibit Iron Accumulation and Enhanced Parkin Expression in the Brain
NeuroMolecular Medicine (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.