Abstract
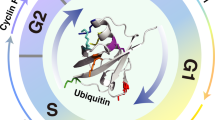
Cyclin D1 regulates G1 cell-cycle progression and is aberrantly expressed in carcinogenesis. Proteasomal degradation of cyclin D1 was highlighted as a cancer chemopreventive mechanism. To understand this mechanism better, residues responsible for degradation and ubiquitination of cyclin D1 were investigated. Eighteen lysines in cyclin D1 had single, double or multiple mutations engineered before transfection into BEAS-2B human bronchial epithelial (HBE) cells to evaluate stabilities after all-trans-retinoic acid (RA) or cycloheximide treatments. Specific mutations stabilized cyclin D1, including substitutions of lysines surrounding the cyclin box domain that inhibited RA-mediated degradation and extended the cyclin D1 half-life. Mutation of all cyclin D1 lysines blocked polyubiquitination. N-terminus (but not C-terminus) modification stabilized cyclin D1. Ubiquitination-resistant mutants preferentially localized cyclin D1 to the nucleus, directly implicating subcellular localization in regulating cyclin D1 degradation. Taken together, these findings uncover specific residues conferring ubiquitination of cyclin D1. These provide a mechanistic basis for proteasomal degradation of cyclin D1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Agami R, Bernards R . (2000). Distinct initiation and maintenance mechanisms cooperate to induce G1 cell cycle arrest in response to DNA damage. Cell 102: 55–66.
Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y . (2004). Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res 24: 2783–2840.
Alt JR, Cleveland JL, Hannink M, Diehl JA . (2000). Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev 14: 3102–3114.
Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G . (1993). Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev 7: 812–821.
Batonnet S, Leibovitch MP, Tintignac L, Leibovitch SA . (2004). Critical role for lysine 133 in the nuclear ubiquitin-mediated degradation of MyoD. J Biol Chem 279: 5413–5420.
Chernov MV, Bean LJ, Lerner N, Stark GR . (2001). Regulation of ubiquitination and degradation of p53 in unstressed cells through C-terminal phosphorylation. J Biol Chem 276: 31819–31824.
Ciechanover A . (1994). The ubiquitin–proteasome proteolytic pathway. Cell 79: 13–21.
Ciechanover A, Ben-Saadon R . (2004). N-terminal ubiquitination: more protein substrates join in. Trends Cell Biol 14: 103–106.
Coulombe P, Rodier G, Bonneil E, Thibault P, Meloche S . (2004). N-terminal ubiquitination of extracellular signal-regulated kinase 3 and p21 directs their degradation by the proteasome. Mol Cell Biol 24: 6140–6150.
DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S et al. (1996). Mapping of the inducible IkappaB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol 16: 1295–1304.
Diehl JA . (2002). Cycling to cancer with cyclin D1. Cancer Biol Ther 3: 226–231.
Diehl JA, Cheng M, Roussel MF, Sherr CJ . (1998). Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev 12: 3499–3511.
Diehl JA, Zindy F, Sherr CJ . (1997). Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin–proteasome pathway. Genes Dev 11: 957–972.
Dmitrovsky E, Sporn MB . (2002). Pharmacology of cancer chemoprevention. In: Bertino J (ed). Encyclopedia of Cancer 2nd edn. Academic Press: St Louis, MO, USA, pp 449–455.
Dragnev KH, Freemantle SJ, Spinella MJ, Dmitrovsky E . (2001). Cyclin proteolysis as a retinoid cancer prevention mechanism. Ann N Y Acad Sci 952: 13–22.
Dragnev KH, Petty WJ, Shah S, Biddle A, Desai NB, Memoli VA et al. (2005). Bexarotene and erlotinib for aerodigestive tract cancer. J Clin Oncol 23: 8757–8764.
Dragnev KH, Pitha-Rowe I, Ma Y, Petty WJ, Sekula D, Murphy B et al. (2004). Specific chemopreventive agents trigger proteasomal degradation of G1 cyclins: implications for combination therapy. Clin Cancer Res 10: 2570–2577.
Fung TK, Yam CH, Poon RY . (2005). The N-terminal regulatory domain of cyclin A contains redundant ubiquitination targeting sequences and acceptor sites. Cell Cycle 4: 1411–1420.
Germain D, Russell A, Thompson A, Hendley J . (2000). Ubiquitination of free cyclin D1 is independent of phosphorylation on threonine 286. J Biol Chem 275: 12074–12079.
Gladden AB, Diehl JA . (2005). Location, location, location: the role of cyclin D1 nuclear localization in cancer. J Cell Biochem 96: 906–913.
Gregory MA, Hann SR . (2000). c-Myc Proteolysis by the ubiquitin–proteasome pathway: stabilization of c-Myc in Burkitt's lymphoma cells. Mol Cell Biol 20: 2423–2435.
King RW, Glotzer M, Kirschner MW . (1996). Mutagenic analysis of the destruction signal of mitotic cyclins and structural characterization of ubiquitinated intermediates. Mol Biol Cell 7: 1343–1357.
Kitareewan S, Pitha-Rowe I, Sekula D, Lowrey CH, Nemeth MJ, Golub TR et al. (2002). UBE1L is a retinoid target that triggers PML/RARα degradation and apoptosis in acute promyelocytic leukemia. Proc Natl Acad Sci USA 99: 3806–3811.
Klotzbucher A, Stewart E, Harrison D, Hunt T . (1996). The ‘destruction box’ of cyclin A allows B-type cyclins to be ubiquitinated, but not efficiently destroyed. EMBO J 15: 3053–3064.
Kobayashi H, Stewart E, Poon R, Adamczewski JP, Gannon J, Hunt T . (1992). Identification of the domains in cyclin A required for binding to, and activation of, p34cdc2 and p32cdk2 protein kinase subunits. Mol Biol Cell 3: 1279–1294.
Langenfeld J, Kiyokawa H, Sekula D, Boyle J, Dmitrovsky E . (1997). Posttranslational regulation of cyclin D1 by retinoic acid: a chemoprevention mechanism. Proc Natl Acad Sci USA 94: 12070–12074.
Lu F, Gladden AB, Diehl JA . (2003). An alternatively spliced cyclin D1 isoform, cyclin D1b, is a nuclear oncogene. Cancer Res 63: 7056–7061.
Ma Y, Feng Q, Sekula D, Diehl JA, Freemantle SJ, Dmitrovsky E . (2005). Retinoid targeting of different D-type cyclins through distinct chemopreventive mechanisms. Cancer Res 65: 6476–6483.
Patel MI, Subbaramaiah K, Du B, Chang M, Yang P, Newman RA et al. (2005). Celecoxib inhibits prostate cancer growth: evidence of a cyclooxygenase-2-independent mechanism. Clin Cancer Res 11: 1999–2007.
Peters JM . (2002). The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol Cell 9: 931–943.
Petty WJ, Dragnev KH, Dmitrovsky E . (2003). Cyclin D1 as a target for chemoprevention. Lung Cancer 41: S155–161.
Pitha-Rowe I, Hassel BA, Dmitrovsky E . (2004a). Involvement of UBE1L in ISG15 conjugation during retinoid-induced differentiation of acute promyelocytic leukemia. J Biol Chem 279: 18178–18187.
Pitha-Rowe I, Petty WJ, Feng Q, Koza-Taylor PH, Dimattia DA, Pinder L et al. (2004b). Microarray analyses uncover UBE1L as a candidate target gene for lung cancer chemoprevention. Cancer Res 64: 8109–8115.
Radu A, Neubauer V, Akagi T, Hanafusa H, Georgescu MM . (2003). PTEN induces cell cycle arrest by decreasing the level and nuclear localization of cyclin D1. Mol Cell Biol 23: 6139–6149.
Scherer DC, Brockman JA, Chen Z, Maniatis T, Ballard DW . (1995). Signal-induced degradation of IκBα requires site-specific ubiquitination. Proc Natl Acad Sci USA 92: 11259–11263.
Solomon DA, Wang Y, Fox SR., Lambeck TC, Giesting S, Lan Z et al. (2003). Cyclin D1 splice variants. Differential effects on localization, RB phosphorylation, and cellular transformation. J Biol Chem 278: 30339–30347.
Spinella MJ, Freemantle SJ, Sekula D, Chang JH, Christie AJ, Dmitrovsky E . (1999). Retinoic acid promotes ubiquitination and proteolysis of cyclin D1 during induced tumor cell differentiation. J Biol Chem 274: 22013–22018.
Zwicker J, Brusselbach S, Jooss KU, Sewing A, Behn M, Lucibello FC et al. (1999). Functional domains in cyclin D1: pRb-kinase activity is not essential for transformation. Oncogene 18: 19–25.
Acknowledgements
This work was supported by National Institutes of Health and National Cancer Institute grants RO1-CA087546 (ED) and RO1-CA111422 (ED) and by the Samuel Waxman Cancer Research Foundation (ED). We thank Ms Ann M Lavanway (Dartmouth College) for assistance with confocal microscopy.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Feng, Q., Sekula, D., Müller, R. et al. Uncovering residues that regulate cyclin D1 proteasomal degradation. Oncogene 26, 5098–5106 (2007). https://doi.org/10.1038/sj.onc.1210309
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210309
Keywords
This article is cited by
-
Tumor expression of survivin, p53, cyclin D1, osteopontin and fibronectin in predicting the response to neo-adjuvant chemotherapy in children with advanced malignant peripheral nerve sheath tumor
Journal of Cancer Research and Clinical Oncology (2018)
-
Cyclin D1 degradation and p21 induction contribute to growth inhibition of colorectal cancer cells induced by epigallocatechin-3-gallate
Journal of Cancer Research and Clinical Oncology (2012)
-
Lysine 269 is essential for cyclin D1 ubiquitylation by the SCFFbx4/αB-crystallin ligase and subsequent proteasome-dependent degradation
Oncogene (2009)
-
An acidic environment changes cyclin D1 localization and alters colony forming ability in gliomas
Journal of Neuro-Oncology (2008)