Abstract
Background:
KIT exon 11 mutations are observed in 60% of gastrointestinal stromal tumours (GIST). Exon 11 codes for residues Tyr568 and Tyr570, which play a major role in signal transduction and degradation of KIT. Our aim was to compare the outcome of patients with deletion of both Tyr568–570 (delTyr) and the most frequent deletion delWK557–558 (delWK).
Methods:
Pathology and clinical characteristics of 68 patients with delTyr (n=26) or delWK (n=42) were reviewed and compared.
Results:
GISTs with delTyr were more frequently extragastric than those with delWK (69 vs 26%, P<0.0005). After curative surgery, median relapse-free survival were 10.8 and 11.1 months for patients with delTyr (n=14) and delWK (n=29), respectively (P=0.92). All patients treated with imatinib for a non-resectable or metastatic GIST had an objective response (n=15) or a stable disease (n=21) as best response, regardless of mutation. Median progression-free survival with imatinib were 21.9 and 18.9 months for patients with GIST with delTyr (n=14) and delWK (n=22), respectively (P=0.43).
Conclusion:
In this large retrospective series, the type of KIT exon 11 mutation was correlated with the origin of GIST, but not with prognosis or response to imatinib.
Similar content being viewed by others
Main
Gastrointestinal stromal tumours (GISTs) are the most frequent mesenchymal tumours of the digestive tract and occur typically in the stomach for two-third or in the small intestine for 25% in most series (Emile et al, 2004). Gain of function mutations of either KIT or platelet-derived growth factor receptor alpha polypeptide (PDGFRA) receptor tyrosine kinases play a critical role in GIST pathogenesis, and are found in 85% of GISTs (Rubin et al, 2007).
Many types of gain of function mutations of KIT and PDGFRA have been described in GISTs, but 60% occurred within the exon 11 of KIT (Corless et al, 2004; Emile et al, 2004), which comprises 33 codons (codons 550–582). The two tyrosines Tyr568 and Tyr570, first residues to be phosphorylated during activation, are consensus sites for binding of Src family kinases and could be implicated in activation of different signalling pathways (Roskoski, 2005). More than 90 mutations of exon 11 have been published, and consist in insertions, substitutions and deletions; however, delWK557–558, in the proximal part of exon 11, is the most frequent, accounting for 8–25% of KIT exon 11 mutations. Others deletions, in the distal part of the exon, include in particular deletions of Tyr568 and/or Tyr570, and may thus have more specific effects on KIT signalling pathways and degradation. Such deletions account for 3–8% of exon 11 mutations in published series (Ernst et al, 1998; Taniguchi et al, 1999; Debiec-Rychter et al, 2004, 2006; Wardelmann et al, 2004; Martin et al, 2005; Penzel et al, 2005; Andersson et al, 2006; Emile et al, 2006; DeMatteo et al, 2008).
After surgical resection, the type of KIT mutations may be a prognostic factor of relapse. KIT exon 11 deletions and deletions affecting codons 557–558 of KIT exon 11 were described to be independent adverse prognostic factors in patients with GIST (Wardelmann et al, 2003; Martin et al, 2005; DeMatteo et al, 2008). Conversely, in another study, GISTs in which the last part of exon 11 (codons 562–579) was deleted were most frequently associated with malignancy than GISTs with deletion of the first part of exon 11 (codons 550–561; Emile et al, 2004, 2006). So, the prognostic value of some types of KIT exon 11 mutations for risk of relapse is still debated. The mutational status of KIT or PDGFRA is predictive of clinical response to imatinib (Glivec, Gleevec, Novartis, Basel, Switzerland) and best results are obtained in patients with GISTs harbouring KIT exon 11 mutations (Heinrich et al, 2003; Debiec-Rychter et al, 2006). Nevertheless, the role of the type of KIT exon 11 mutations for the response and survival under imatinib remains to be determined.
Thus, to better understand the prognostic significance of the type of KIT exon 11 deletions, we have compared the clinical characteristics and outcome of patients with GIST and deletion of both Tyr568 and Tyr570 with the most frequent deletion of KIT exon 11, delWK557–558.
Materials and methods
Patient selection
From database of two French pathology departments which detect KIT and PDGFRA mutations in routine practice (Ambroise Pare Hospital, Boulogne; Bergonie Institute, Bordeaux, France), we searched retrospectively for all consecutive patients with GIST and with either delWK or deletions including both residues Tyr568 and Tyr570 (delTyr). Mutations within exon 9, 11, 13 and 17 of KIT and within exon 12 and 18 of PDGFRA were detected as previously described (Emile et al, 2002, 2004; Heinrich et al, 2003; Hostein et al, 2006).
Pathology
All samples were obtained before treatment with imatinib. Paraffin-embedded samples were independently analysed by at least two pathologists. For resected GIST, largest tumour diameter and mitotic count per 50 high-power fields (HPF) were evaluated after surgery in each case, as recommended by international criteria and used to evaluate the risk of GIST malignancy (Fletcher et al, 2002; Miettinen and Lasota, 2006). Immunohistochemistry was performed with anti-CD117 (A-4502, polyclonal; DAKO, Copenhagen, Denmark).
Clinical data and survival analysis
Medical records of all patients were retrospectively reviewed. Response rates to imatinib were evaluated by spiral computerised tomography according to the RECIST criteria. The relapse-free survival (RFS) was defined as the time between the date of curative surgery and the date of relapse. The progression-free survival (PFS) was defined as the time between the first day of imatinib and the date of progression or death. Overall survival (OS) under imatinib was defined as the time between the first day of imatinib and the date of death or last follow-up.
Statistical analysis
Results are expressed as medians and ranges. The cut-off date for the final analysis was 15 January 2008. We used Student's t-test to compare quantitative data in univariate analyses and χ2-tests were used for qualitative data. We estimated RFS, PFS and OS using the Kaplan–Meier method, and we used log-rank tests to compare the survival curves (Kaplan and Meier, 1958). SAS software v 9.1 (SAS Institute Inc., Cary, NC, USA) was used for all statistical analysis.
Results
Mutation, clinical and pathologic characteristics
A total of 68 patients with GIST, diagnosed between 1985 and 2007, and all CD117 positive, were retrieved. DelWK and delTyr accounted for 18% (34/185) and 10% (19/185) of KIT exon 11 mutations in Ambroise Paré's series, respectively. Out of the 26 delTyr mutations, 8 also involved both amino acids 557 and 558, and one involved the amino acid 558. Details of delTyr mutations are summarised in Figure 1.
GISTs with delTyr were more frequently extragastric than those with delWK (69 vs 26%, P<0.0005), whereas other clinical and tumour characteristics were not different (Table 1). After exclusion of the 8 GISTs with delTyr including the 2 amino acids 557 and 558, GISTs with delTyr (n=18) were still more frequently extragastric (P=0.0031).
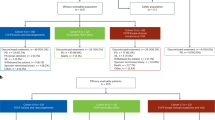
Distribution of patients according to the outcome and the type of KIT exon 11 deletion is described in Figure 2.
Relapse-free survival
Mitotic count, tumour size and risk classifications were not different between patients with delWK and those with delTyr (Table 2). At the date of cut-off, median time since curative surgery was 5.1 years (range 0.4–21.9 years). Three patients with delWK and two patients with delTyr had been included in an adjuvant prospective trial with imatinib, and were excluded of RFS analysis. Median RFS were 11.1 months (95% CI: 9.4–66.6) and 10.8 months (95% CI: 7.1–57.7; P=0.92; Figure 3A), respectively. Results were not modified after exclusion of the eight GISTs with delTyr including the two amino acids 557 and 558 (P=0.45).
Objective response and survival under imatinib
During follow-up, 22 patients with delWK and 14 patients with delTyr received imatinib (Figure 2). Out of these 36 patients, 26 (72%) had been included and evaluated in a prospective trial. At the date of cut-off, median time since imatinib beginning was 4.7 years (range 0.7–6.8 years).
Objective responses to imatinib were not different between patients with delWK and those with delTyr (Table 3). Median PFS under imatinib were 18.9 months (95% CI: 12.6– ) for patients with delWK and 21.9 months (95% CI: 16.1–37.4) for patients with delTyr (P=0.43; Figure 3B). Median OS since imatinib beginning were 31.4 months (95% CI: 19.7–) and 38.6 months (95% CI: 35.4–45.3; P=0.31; Figure 3C), respectively. After exclusion of the 8 GISTs with delTyr including the two amino acids 557 and 558, results were not modified for median PFS (P=0.60) and OS (P=0.39).
Discussion
KIT exon 11 mutations are present in the majority of GISTs. Many different types of mutations have been published, some of which delete residues Tyr568 and Tyr570, which play an important role in KIT signal transduction. Thus, we compared these mutations (delTyr) with the most frequent mutation of KIT exon 11 in GISTs (delWK557–558). Analysis of our large series of patients shows that GISTs with delWK are mainly gastric, whereas GISTs with delTyr are mainly intestinal. However, GISTs with these mutations had identical prognosis after curative surgery and response to imatinib treatment.
Previous studies described that the GIST's location was associated with type of mutation. GISTs with KIT exon 9 mutation arise predominantly in small intestine and colon, and those with PDGFRA mutations most often originate from the stomach (Emile et al, 2004; Wardelmann et al, 2004; Penzel et al, 2005). Our results show that GISTs with delTyr arise in small intestine, colon or rectum in about 70% of cases, whereas those with delWK557–558 occur in stomach in about 75% of cases, and this difference was highly significant. This suggests possible different types of oncogenic events driving KIT mutations in the different parts of the digestive tract.
Recently, some studies reported that GISTs with delWK557–558 have an increased risk of relapse after curative surgery (Wardelmann et al, 2003; Martin et al, 2005; DeMatteo et al, 2008). In our study, GISTs with delWK557–558 and GISTs with delTyr did not differ for the risk of relapse after curative surgery and both convey a poor prognosis. According to tumour location, independently of the risk stage, a relapse occurred in 56% (14/25) and 75% (3/4) of gastric GISTs with delWK and delTyr, and in 40% (2/5) and 66.7% (6/9) of intestinal GISTs, respectively. So, GISTs with these mutations seem to have the same worse prognosis and gastric GIST with exon 11 mutations may be of the same poor prognosis as small bowel or large bowel GIST actually.
The outcome of non-resectable and metastatic GISTs with delWK and delTyr under imatinib is similar in terms of response rates, PFS and OS. All patients included in our study had an objective response or a stable disease under imatinib, and median PFS were of about 20 months. These results are concordant with results of published phase III studies (Heinrich et al, 2003; Debiec-Rychter et al, 2006).
In this large retrospective series, the type of KIT exon 11 mutation differed according to primary site, with delWK originating from the stomach, whereas those with delTyr from the intestine. However, GISTs with these mutations had the same prognosis after curative surgery and under imatinib.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Andersson J, Bümming P, Meis-Kindblom JM, Sihto H, Nupponen N, Joensuu H, Odén A, Gustavsson B, Kindblom LG, Nilsson B (2006) Gastrointestinal stromal tumors with exon 11 deletions are associated with poor prognosis. Gastroenterology 130: 1573–1581
Corless CL, Fletcher JA, Heinrich MC (2004) Biology of gastrointestinal tumors. J Clin Oncol 22: 3813–3825
Debiec-Rychter M, Dumez H, Judson I, Wasag B, Verweij J, Brown M, Dimitrijevic S, Sciot R, Stul M, Vranck H, Scurr M, Hagemeijer A, van Glabbeke M, van Oosterom AT, EORTC Soft Tissue and Bone Sarcoma Group (2004) Use of c-KIT/PDGFRA mutational analysis to predict the clinical response to imatinib in patients with advanced gastrointestinal stromal tumours entered on phase I and II studies of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 40: 689–695
Debiec-Rychter M, Sciot R, Le Cesne A, Schlemmer M, Hohenberger P, van Oosterom AT, Blay JY, Leyvraz S, Stul M, Casali PG, Zalcberg J, Verweij J, Van Glabbeke M, Hagemeijer A, Judson I, EORTC Soft Tissue and Bone Sarcoma Group; The Italian Sarcoma Group, Australasian Gastrointestinal Trials Group (2006) KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer 46: 1093–1103
DeMatteo RP, Gold JS, Saran L, Gönen M, Liau KH, Maki RG, Singer S, Besmer P, Brennan MF, Antonescu CR (2008) Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer 112: 608–615
Emile JF, Lemoine A, Bienfait N, Terrier P, Azoulay D, Debuire B (2002) Length analysis of polymerase chain reaction products: a sensitive and reliable technique for the detection of mutations in KIT exon 11 in gastrointestinal stromal tumors. Diagn Mol Pathol 11: 107–112
Emile JF, Tabone-Eglinger S, Théou-Anton N, Lemoine A (2006) Prognostic value of KIT exon 11 deletions in GISTs. Gastroenterology 131: 976–977
Emile JF, Théou N, Tabone S, Cortez A, Terrier P, Chaumette MT, Julié C, Bertheau P, Lavergne-Slove A, Donadieu J, Barrier A, Le Cesne A, Debuire B, Lemoine A, Groupe d'étude des GIST (2004) Clinicopathologic, phenotypic, and genotypic characteristics of gastrointestinal mesenchymal tumors. Clin Gastroenterol Hepatol 2: 597–605
Ernst SI, Hubbs AE, Przygodzki RM, Emory TS, Sobin LH, O'Leary TJ (1998) KIT mutation portends poor prognosis in gastrointestinal stromal/smooth muscle tumors. Lab Invest 78: 1633–1636
Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW (2002) Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 33: 459–465
Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ, Kiese B, Eisenberg B, Roberts PJ, Singer S, Fletcher CD, Silberman S, Dimitrijevic S, Fletcher JA (2003) Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumors. J Clin Oncol 21: 4342–4349
Hostein I, Longy M, Gastaldello B, Geneste G, Coindre JM (2006) Detection of a new mutation in KIT exon 9 in a gastrointestinal stromal tumor. Int J Cancer 118: 2089–2091
Kaplan EL, Meier P (1958) Nonparametric estimation for incomplete observations. J Am Stat Assoc 53: 457–481
Martin J, Poveda A, Llombart-Bosch A, Ramos R, Lopez-Guerrero JA, Garcia del Muro J, Maurel J, Calabuig S, Gutierrez A, Gonzalez de Sande JL, Martinez J, De Juan A, Lainez N, Losa F, Alija V, Escudero P, Casado A, Garcia P, Blanco R, Buesa JM, Spanish Group for Sarcoma Research (2005) Deletions affecting codons 557–558 of the c-KIT gene indicate a poor prognosis in patients with completely resected gastrointestinal stromal tumors: a study by the Spanish Group for Sarcoma Research (GEIS). J Clin Oncol 23: 6190–6198
Miettinen M, Lasota J (2006) Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 23: 70–83
Penzel R, Aulmann S, Moock M, Schwarzbach M, Rieker RJ, Mechtersheimer G (2005) The location of KIT and PDGFRA gene mutaions in gastrointestinal stromal tumors is site and phenotype associated. J Clin Pathol 58: 634–639
Roskoski R (2005) Structure and regulation of Kit protein-tyrosine kinase – the stem cell factor receptor. Biochem Biophys Res Commun 338: 1307–1315
Rubin BP, Heinrich MC, Corless CL (2007) Gastrointestinal stromal tumor. Lancet 369: 1731–1741
Taniguchi M, Nishida T, Hirota S, Isozaki K, Ito T, Nomura T, Matsuda H, Kitamura Y (1999) Effect of c-kit mutation on prognosis of gastrointestinal stromal tumors. Cancer Res 59: 4297–4300
Wardelmann E, Hrychyk A, Merkelbach-Bruse S, Pauls K, Goldstein J, Hohenberger P, Losen I, Manegold C, Büttner R, Pietsch T (2004) Association of platelet-derived growth factor receptor α mutations with gastric primary site and epithelioid or mixed morphology in gastrointestinal stromal tumors. J Mol Diagn 6: 197–204
Wardelmann E, Losen I, Hans V, Neidt I, Speidel N, Bierhoff E, Heinicke T, Pietsch T, Büttner R, Merkelbach-Bruse S (2003) Deletion of Tryp-557 and Lys-558 in the juxtamembrane domaine of the c-kit protooncogene is associated with metastatic behavior of gastrointestinal stromal tumors. Int J Cancer 106: 887–895
Acknowledgements
We acknowledge all our colleagues who helped us for this study. This works was supported by grants from PHRC03055, BQR2007 Versailles SQY University and unrestricted grants from Novartis Oncology. Jean-Baptiste Bachet is a fellow of AERIO (association d'enseignement et de recherche des internes en oncologie), with financial support from Janssen-Cilag.
Author information
Authors and Affiliations
Corresponding author
Additional information
Financial disclosures: Consultant or advisory relationship: Axel Le Cesne: Novartis and Pfizer; Binh Bui: Novartis and Pharmamar; Jean-Yves Blay: Novartis, Pfizer and GSK; Jean-François Emile: Novartis.
Honoraria: Axel Le Cesne: Novartis and Pfizer; Binh Bui: Novartis and Pharmamar; Jean-Yves Blay: Novartis, Pfizer and GSK; Jean-François Emile: Novartis.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Bachet, JB., Hostein, I., Le Cesne, A. et al. Prognosis and predictive value of KIT exon 11 deletion in GISTs. Br J Cancer 101, 7–11 (2009). https://doi.org/10.1038/sj.bjc.6605117
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6605117
Keywords
This article is cited by
-
Personalized radiomics signature to screen for KIT-11 mutation genotypes among patients with gastrointestinal stromal tumors: a retrospective multicenter study
Journal of Translational Medicine (2023)
-
Durable Disease Control with Regorafenib in a Patient with Metastatic KIT-Mutated Colorectal Cancer
Journal of Gastrointestinal Cancer (2021)
-
Prognostic value of mutational characteristics in gastrointestinal stromal tumors: a single-center experience in 275 cases
Medical Oncology (2014)
-
Coexistence of gastrointestinal stromal tumors and gastric adenocarcinomas
Tumor Biology (2013)
-
Molecular characterization of an Italian series of sporadic GISTs
Gastric Cancer (2013)