Abstract
Vimentin, nuclear factor-κB (NF-κB) p105, fascin, E-cadherin, TGF-β, Par6 and atypical PKC are molecular markers that play an important role in cell differentiation. Herein, we investigate their prognostic impact in primary non-small-cell carcinoma (NSCLC). Tumour tissue samples from 335 resected patients with stage I–IIIA were used. Tissue microarrays were constructed from duplicate cores of both neoplastic cells and stromal cells and were immunohistochemically evaluated. In univariate analyses, high tumour epithelial cell expressions of NF-κB p105 (P=0.02) and E-cadherin (P=0.03) were positive prognostic indicators for disease-specific survival (DSS), whereas high tumour epithelial cell expression of vimentin (P=0.001) was a negative prognostic indicator. High expression of NF-κB p105 (P=0.001) and Par6 (P=0.0001) in the stromal compartment correlated with a good prognosis. In multivariate analyses, the tumour epithelial cell expression of NF-κB p105 (P=0.0001) and vimentin (P=0.005) and the stromal cell expression of NF-κB p105 (P=0.007) and Par6 (P=0.0001) were independent prognostic factors for DSS. High expression of NF-κB p105 and low expression of vimentin in tumour epithelial cells are independent predictors of better survival in primary NSCLC. In stromal cells, high expressions of NF-κB p105 and Par6 are both favourable independent prognostic indicators.
Similar content being viewed by others
Main
Regarded as a rare malignancy as recently as 1945, lung cancer is now the most common cause of cancer death worldwide, with an estimated annual incidence of more than 1.2 million cases and a mortality of more than 1.1 million cases per year (Jemal et al, 2002). Despite recent advances in chemotherapy, radiation therapy and surgery, the overall survival for lung cancer patients is still less than 15%. This is particularly related to the large population with advanced stage of disease at diagnosis and the poor treatment effects in metastatic disease (Toloza and D’Amico, 2005). Non-small-cell carcinoma (NSCLC) accounts for at least 80% of all lung tumours (Ihde and Minna, 1991) and is subdivided into four major histological groups: squamous cell carcinoma (SCC), adenocarcinoma (AC), bronchioalveolar carcinoma (BAC) and large-cell carcinoma (LCC) (Travis et al, 2004).
A major part of patients diagnosed with cancer die as a result of cancer metastases resistant to conventional therapy (Fidler, 2002), yet the mechanisms regulating tissue changes from benign to invasive and finally to metastatic carcinoma remain fairly vague (Chaffer et al, 2006). A better understanding of these mechanisms will yield major contributions for improving both diagnostic and therapeutic procedures. A range of mechanisms used by oncogenes or tumour suppressor genes during malignant transformation has been revealed. Some of these are accompanied by morphological changes.
Herein, we investigate the expression of a panel of seven markers associated with tumorigenesis in NSCLC both in epithelial tumour cells and in tumour stroma. Our study included the phosphorylated nuclear factor-κB (NF-κB) subunit p105 (NF-κB p105), vimentin, E-cadherin, atypical PKC (aPKC), Par6, fascin and transforming growth factor-β (TGF-β). Their prognostic value has been assessed using high-throughput tissue microarray (TMA) analyses.
Patients and methods
Patients and clinical samples
Primary tumour tissues from anonymised patients diagnosed with NSCLC pathologic stage I–IIIA (Greene et al, 2002) at the University Hospital of North Norway (UNN) and Nordland Central Hospital (NLSH) from 1990 through 2004 were used in this study. In total, 371 patients were registered from the hospital database. Of these, 36 patients were excluded from the study due to (i) radiotherapy or chemotherapy before surgery (n=10); (ii) other malignancies within 5 years before the NSCLC diagnosis (n=13); and (iii) inadequate paraffin-embedded fixed tissue blocks (n=13). Thus, 335 patients with complete medical records and adequate paraffin-embedded tissue blocks were eligible.
This report includes follow-up data as of 30 September 2005. The median follow-up was 96 (range 10–179) months. Complete demographic and clinical data were collected retrospectively. Formalin-fixed and paraffin-embedded tumour specimens were obtained from the archives of the Departments of Pathology at UNN and NLSH. The tumours were staged according to the International Union Against Cancer's TNM classification and histologically subtyped and graded according to the World Health Organization (Travis et al, 2004). Regarding N status, ipsilateral peribronchial or hilar nodes and intrapulmonary nodes are defined as N1, whereas N2 includes ipsilateral mediastinal or subcarinal nodes.
Microarray construction
All lung cancer cases were histologically reviewed by two pathologists (SAS and KAS), and the most representative areas of viable invasive carcinoma tissue (epithelial cells) and surrounding tumour stroma from central parts within the tumour were carefully selected and marked on the haematoxylin and eosin slides and sampled for the TMA blocks. The TMAs were assembled using a tissue-arraying instrument (Beecher Instruments, Silver Springs, MD, USA). The detailed methodology has been reported earlier (Bremnes et al, 2006).
TMA represents a practical tool for studying a large number of biological markers that may have significant therapeutic effects. Studying whole tissue sections is, however, still needed to increase the value of the TMA results before therapeutical implementation. Briefly, we used a 0.6-mm diameter stylet, and the study specimens were routinely sampled in duplication from epithelial cancer cells and from tumour-surrounding stroma intervening malignant epithelial areas. Normal lung tissue localised distant from the primary tumour were used as negative controls.
To include all core samples, eight tissue array blocks were constructed. Multiple 5-μm sections were cut with a Micron microtome (HM355S) and stained by specific antibodies for immunohistochemistry (IHC) analysis.
Immunohistochemistry
The applied antibodies had been subjected to in-house validation by the manufacturer for IHC analysis on paraffin-embedded material. For the antibodies used in the study, see Table 1. For staining with fascin and NF-κB p105, sections were deparaffinised with xylene and rehydrated with ethanol. Antigen retrieval was performed by placing the specimen in 0.01 mol l−1 citrate buffer at pH 6.0 and exposed to two repeated microwave heatings of 10 min at 450 W. The DAKO EnVision+System-HRP (DAB) kit was used for endogen peroxidase blocking. Primary antibodies for fascin and NF-κB p105 were incubated for 30 min at room temperature. For staining with the remaining antibodies, the slides were transferred to the Ventana Benchmark, XT automated slide stainer (Ventana Medical System, Illkirch, France). The DAKO EnVision+ System-HRP (DAB) kit was used to visualise the antigens for all stains. This yielded a brown reaction product at the site of the target antigen. Tissue sections were incubated with primary antibodies recognising vimentin, E-cadherin, Par6, aPKC and TGF-β. Primary antibodies were incubated at 37°C (vimentin 24 min, E-cadherin 32 min, aPKC 28 min, Par6 52 min and TGF-β 28 min). As negative staining controls, the primary antibodies were replaced with the primary antibody diluent. Finally, all slides were counterstained with haematoxylin to visualise the nuclei. For each antibody, including negative controls, all TMA stainings were performed in a single experiment (Figure 1).
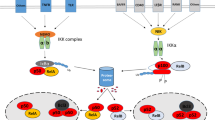
Simplified schematic illustration of signals regulating cell differentiation. (A) Polarised normal epithelium cells with tight junctions between cells resting on the basement membrane. (B) Different risk factors (including cigarette smoke, toxins and genetic factors) can cause activation of different oncogenes, which regulate cell differentiation. Activation of receptor tyrosine kinases (RTKs) is known to play a role in inducing mesenchymal phenotype. (C) Unpolarised spindle-shaped epithelial cells with loss of tight junctions and upregulation of mesenchymal markers like vimentin and fascin. TGF-β=transforming growth factor-β; TGF-βR=transforming growth factor-beta receptor; RAS=rat sarcoma oncogene; NF-κB=nuclear factor-κB; AKT/PKB=AKT/protein kinase B; Par6=partitioning-defective protein-6; aPKC=atypical protein kinase C.
Scoring of IHC
By light microscopy, representative viable tissue sections were scored semiquantitatively for cytoplasmic or membranous staining (Figure 2). The dominant staining intensity in both tumour epithelial cells and stromal cells was scored as 0=negative; 1=weak; 2=intermediate; and 3=strong. The cell density of the stroma was scored as 1=low density; 2=intermediate density; and 3=high density. All samples were anonymised and independently scored by two pathologists (SAS and KAS). In case of disagreement, the slides were re-examined and a consensus was reached by the observers. When assessing one variable for a given core, the observers were blinded to the scores of the other variables and to outcome. To evaluate the interobserver agreement with respect to IHC scoring, 100 consecutive tumour epithelial cell cores and tumour stroma cores, stained for two rabbit polyclonal markers (VEGF-C and VEGFR-3), were examined (Donnem et al, 2007). The mean correlation coefficient (r) was 0.95 (range 0.93–0.98) and was assessed for both antibodies in both tumour epithelial and stromal areas.
Mean score for duplicate cores from each individual was calculated separately in tumour epithelial cells and stroma, and a high expression in tumour epithelial cells was defined as score ⩾2. Stromal expression was calculated by summarising density score (1–3) and intensity score (0–3) before categorising into low and high expression. High expression in stroma was defined as score ⩾4.
Statistical methods
All statistical analyses were performed using the statistical package SPSS (Chicago, IL, USA), version 14. The χ2 test and Fisher's Exact test were used to examine the association between molecular marker expression and various clinicopathological parameters. Univariate analysis was performed by using the Kaplan–Meier method, and statistical significance between survival curves was assessed by the log rank test. Disease-specific survival (DSS) was determined from the date of surgery to the time of lung cancer death. To assess the independent value of different pre-treatment variables on survival, in the presence of other variables, multivariate analysis was carried out using the Cox proportional hazards model. Only variables of significant value from the univariate analysis were entered into the Cox regression analysis. Probability for stepwise entry and removal was set at 0.05 and 0.1, respectively.
Ethics clearance
The National Data Inspection Board and The Regional Committee for Research Ethics approved the study.
Results
Patient data
Demographical, clinical and histopathological variables are shown in Table 1. The median age was 67 years (range 28–85) and 75% of the patients were male individuals. The patient population of 335 cases represented the four major subtypes of NSCLC with 191 SCCs, 95 ACs, 31 LCCs and 18 BACs. Owing to nodal metastases or non-radical surgical margins, 18% (59 patients) received postoperative radiotherapy.
Expression pattern and correlations with clinicopathological variables
Our IHC analyses included the phosphorylated NF-κB p105, vimentin, E-cadherin, aPKC, Par6, fascin and TGF-β. All the investigated markers except E-cadherin were expressed in the cytoplasm of tumour epithelial cells. E-cadherin showed a membranous staining. On the basis of morphological criteria, pneumocytes in control cores from normal lung tissue, distant from the primary tumour, generally showed weak positive immunostaining.
In tumour stroma and in control cores, inflammatory cells (macrophages, lymphocytes, granulocytes and plasma cells) and endothelial cells frequently showed positive staining for NF-κB p105, vimentin and Par6, whereas fibroblast-like cells only occasionally presented positive staining. No stromal cell staining was observed for E-cadherin, fascin, TGF-β and aPKC.
Expression of the investigated markers in tumour epithelial cells or stroma did not correlate with clinical performance status, vascular infiltration or histological subgroups. Expression of vimentin and Par6 in tumour epithelial cells correlated significantly (r=0.2, P<0.001), as did vimentin and tumour differentiation (r=−0.1, P=0.01). High expression of vimentin was seen in 61% of poorly differentiated tumours, 39% of moderately differentiated tumours and no expression was seen in well-differentiated tumours. Even among the patient group with poorly differentiated tumours, high tumour epithelial cell vimentin expression tended to correlate with poor survival (P=0.08). Further, a significant correlation was seen between tumour epithelial expression of aPKC and Par6 (r=0.2, P<0.001).
Univariate analysis
In addition to the statistically significant clinical variables (Table 2), tumour epithelial cell expression of NF-κB p105 (P=0.02), vimentin (P=0.001) and E-cadherin (P=0.03), and stromal cell expression of NF-κB p105 (P=0.001) and Par6 (P=0.0001) were prognostic indicators for DSS in univariate analyses (Table 3; Figures 3 and 4). Stratifying the cases based on histology revealed that the significance of better survival for high tumour epithelial NF-κB p105 and vimentin expression was limited to ACs (P=0.03 and P=0.0004, respectively) and stromal NF-κB p105 and Par6 expression to SCCs (P=0.001 and P=0.0009, respectively). There was no significant association between DSS and tumour epithelial cell expression of aPKC (P=0.2), Par6 (P=0.7), fascin (P=0.4), TGF-β (P=0.1) or stromal cell expression of vimentin (P=0.3) (Table 3).
Multivariate Cox proportional hazards analysis
All significant clinicopathological and molecular variables from the univariate analyses were entered into the multivariate analysis. Data are presented in Table 4. Tumour epithelial cell expression of NF-κB p105 (P=0.001) and vimentin (P=0.005), stromal cell expression of NF-κB p105 (P=0.007) and Par6 (P=0.0001), and the clinicopathological variables T stage (P=0.02), N stage (P=0.0001) and performance status (P=0.03) were all independent prognostic factors for survival. High tumour epithelial cell expression of E-cadherin (P=0.05), histological differentiation (P=0.05) and vascular infiltration (P=0.06) tended towards a statistical significance.
Discussion
Using high-throughput TMA analyses, an established method for assessing protein expression across large sets of lung cancer tissues (Bremnes et al, 2002), we sought to determine the prognostic impact of a panel of seven molecular markers associated with tumorigenesis. High stromal expression of NF-κB p105 and Par6 as well as high epithelial tumour cell expression of NF-κB p105 showed an independent positive correlation with DSS. In contrast, vimentin expression in tumour epithelial cells was an independent negative prognostic indicator for DSS.
Immunohistochemistry as a method for detecting protein expression in paraffin-embedded tissue has been shown to be both highly sensitive and specific (Zafrani et al, 2000), yet the specificity of an immunohistochemical test would never exceed the specificity of the antibody provided by the manufacturer. Nevertheless, an additional possible source of error can still be the biological variation of protein expression in different areas of tumour tissue. Nonetheless, this source of bias can be reduced by increasing the number of examined tissue as in this study.
Stromal–epithelial interactions are considered critical for regulating tissue development and for the maintenance of tissue homoeostasis (Kass et al, 2007). Consequently, it seems essential also to study the tumour stroma and its different molecular markers to be able to understand the mechanism of tumour metastases. In this study, the term ‘stroma’ comprises all groups of non-epithelial cells and structures intervening between islands of tumour epithelial cells, that is mesenchymal cells (fibroblasts and fibroblast-like cells), leukocytes, macrophages, endothelial cells and extracellular matrix (ECM) including collagen. Investigating the stromal compartment can, however, be complicated as the stroma is not static. Cellular and ECM compositions evolve over time, adapting to changes related to the surrounding epithelial cells (Kass et al, 2007).
Stromal cells appear, in some instances, to support growth and motility of tumour cells (Costea et al, 2006), and in other instances to be part of the microenvironment in preventing tumour cell invasion, in concert with the assumed function of the immune system. Thus, stromal cells have rather complex and controversial roles during the ‘abnormal’ condition of tumorigenesis. Hence, it is of great interest that a high stromal expression of NF-κB p105 and Par6 correlates favourably with patient survival.
Nuclear factor-κB is a group of proteins that control inflammation, cell survival, transformation, proliferation, angiogenesis and apoptosis (Aggarwal, 2004). It is normally retained in the cytoplasm in an inactive state through interaction with inhibitor κB (IκB) (Karin et al, 2002). Degradation of the IκB proteins results in the liberation of NF-κB, allowing nuclear translocation and the activation of target genes, including Snail and Bcl-2 (Guarino, 2007). NF-κB is activated in a range of human cancers and is assumed to promote tumorigenesis (Radisky and Bissell, 2007). Five mammalian NF-κB proteins have been identified: p65 (RelA), NF-κB1 (p50 and its precursor p105), NF-κB2 (p52 and its precursor p100), c-rel and RelB (Ghosh et al, 1990). These bind to DNA as homo- or heterodimers.
Havard et al (2005) observed high levels of NF-κB p105 precursor in cell lines derived from human cervical cancers associated with HPV16 infection and in keratinocytes transfected by oncogenes. In another study, Ishikawa et al (1998) observed that NF-κB p105−/− mice showed abnormalities such as inflammation in lungs and liver, myeloid hyperplasia in bone marrow and splenomegaly (Weih et al, 1994). In a study that included 45 patients with NSCLC, Zhang et al (2006) found overexpression of NF-κB p50 to indicate an unfavourable overall survival in NSCLC patients. To our knowledge, the prognostic significance of the precursor NF-κB p105 in both tumour epithelial and stromal cells of NSCLC has hitherto not been reported. In our study, NF-κB p105 expressions in both tumour epithelial and stromal cells were favourable independent prognostic indicators for survival.
Par6 and aPKC have emerged as central players in the regulation of cell polarity and the asymmetric division of cells (Etienne-Manneville and Hall, 2003). Par6 is an adapter protein that engages in many protein–protein interactions regulated to control cell polarity (Thiery and Huang, 2005). In previous cancer cell line studies, Regala et al (2005) categorised aPKC-iota as an oncogene in NSCLC. Through PubMed searches, we could not identify studies investigating the prognostic significance of Par6 and aPKC-iota expression in resected primary NSCLC tissues. In this study, we found high Par6 stromal cell expression to be associated with an improved prognosis, whereas tumour epithelial cell expression of Par6 and aPKC-iota did not show any prognostic relevance. These results indicate that an observed significance of protein expression in cell lines in vitro does not necessarily have the same impact in vivo. The interplay with the ECM may be essential in explaining the discrepancy of these results.
Cadherin-mediated cell–cell adhesion is considered a suppressor of cancer cell invasion in vitro (Behrens et al, 1993). Loss of E-cadherin has previously been attributed to an unfavourable prognostic significance in bladder cancer (Baumgart et al, 2007). Low E-cadherin expression in NSCLC tumours has been reported in several studies (Liu et al, 2001) to be associated with a more ‘aggressive’ behaviour of tumour epithelial cells and with a worse prognosis. Our results on E-cadherin expression in tumour epithelial cells are consistent with these findings.
We further examined the significance of fascin and vimentin expression in NSCLC. Fascin is an actin-bundling and crosslinking protein that binds to preformed filaments and regulates their organisation and stability. It is presumed to regulate cortical cell membrane protrusions (Kureishy et al, 2002). Its overexpression is proposed to increase the motility of epithelial cells (Adams, 2004). The available data on fascin expression and its significance in NSCLC are scarce. In a recent study by Pelosi et al (2003) in 220 stage I NSCLC patients, high tumour cell expression of fascin emerged as an independent predictor of lymph node metastases and poor survival. Our data did not, however, show any correlation between tumour expression of fascin and lymph node metastases or survival.
Vimentin is a structural protein from cells of mesenchymal origin (Leader et al, 1987). Its expression is higher in migratory epithelial cells and may contribute to the migratory and invasive phenotype of metastatic cells (Bindels et al, 2006). Significant correlations between high vimentin tumour cell expression and poor prognosis have previously been reported both in hepatocellular (Hu et al, 2004) and breast carcinoma (Dandachi et al, 2001). Consistent with the latter reports, we found high tumour epithelial cell vimentin expression to be an independent prognosticator for poor survival in resected NSCLC patients. In contrast, Pelletier et al (2001) in 113 resected NSCLC patients found no such correlation.
Transforming growth factor-β is a multifunctional cytokine that plays a central role in signalling networks regulating cell growth, differentiation, adhesion and apoptosis, and is one of the most potent and better-studied markers inducing mesenchymal phenotype in epithelial cells (Janda et al, 2002). There are indications that TGF-β-dependent loss of tight junctions is mediated through Par6 phosphorylation by the TGF-β receptor and is probably independent of Smad-mediated transcriptional responses (Ozdamar et al, 2005). In a recent study, von Rahden et al (2006) observed a correlation between high TGF-β tumour cell expression and a poor prognosis in oesophageal AC. In NSCLC tumours (Boldrini et al, 2000; Hasegawa et al, 2001; Saji et al, 2003), there are conflicting data regarding associations between TGF-β expression and survival. In this study, no significant association was seen between TGF-β tumour cell expression and overall survival.
In summary, we detected an unanticipated behaviour of tumour stromal cells and their impact on survival. Hence, the interaction between tumour stromal cells and tumour epithelial cells plays an important role in the process of cell invasion and metastases, yet many nuances of this interaction are still not clear. Our data give rise to additional investigation, which may provide the foundation for generating more effective therapeutic strategies not only in NSCLC but also in other malignancies.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Adams JC (2004) Roles of fascin in cell adhesion and motility. Curr Opin Cell Biol 16: 590–596
Aggarwal BB (2004) Nuclear factor-kappaB: the enemy within. Cancer Cell 6: 203–208
Baumgart E, Cohen MS, Silva NB, Jacobs MA, Wotkowicz C, Rieger-Christ KM, Biolo A, Zeheb R, Loda M, Libertino JA, Summerhayes IC (2007) Identification and prognostic significance of an epithelial–mesenchymal transition expression profile in human bladder tumors. Clin Cancer Res 13: 1685–1694
Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, Birchmeier W (1993) Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol 120: 757–766
Bindels S, Mestdagt M, Vandewalle C, Jacobs N, Volders L, Noel A, Van Roy F, Berx G, Foidart JM, Gilles C (2006) Regulation of vimentin by SIP1 in human epithelial breast tumor cells. Oncogene 25: 4975–4985
Boldrini L, Calcinai A, Samaritani E, Pistolesi F, Mussi A, Lucchi M, Angeletti CA, Basolo F, Fontanini G (2000) Tumour necrosis factor-alpha and transforming growth factor-beta are significantly associated with better prognosis in non-small cell lung carcinoma: putative relation with BCL-2-mediated neovascularization. Br J Cancer 83: 480–486
Bremnes RM, Camps C, Sirera R (2006) Angiogenesis in non-small cell lung cancer: the prognostic impact of neoangiogenesis and the cytokines VEGF and bFGF in tumours and blood. Lung Cancer 51: 143–158
Bremnes RM, Veve R, Gabrielson E, Hirsch FR, Baron A, Bemis L, Gemmill RM, Drabkin HA, Franklin WA (2002) High-throughput tissue microarray analysis used to evaluate biology and prognostic significance of the E-cadherin pathway in non-small-cell lung cancer. J Clin Oncol 20: 2417–2428
Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED (2006) Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res 66: 11271–11278
Costea DE, Kulasekara K, Neppelberg E, Johannessen AC, Vintermyr OK (2006) Species-specific fibroblasts required for triggering invasiveness of partially transformed oral keratinocytes. Am J Pathol 168: 1889–1897
Dandachi N, Hauser-Kronberger C, More E, Wiesener B, Hacker GW, Dietze O, Wirl G (2001) Co-expression of tenascin-C and vimentin in human breast cancer cells indicates phenotypic transdifferentiation during tumour progression: correlation with histopathological parameters, hormone receptors, and oncoproteins. J Pathol 193: 181–189
Donnem T, Al Saad S, Al Shibli K, Delghandi MP, Persson M, Nilsen MN, Busund LT, Bremnes RM (2007) Inverse prognostic impact of angiogenic marker expression in tumor cells vs stromal cells in non small cell lung cancer. Clin Cancer Res 13: 6649–6657
Etienne-Manneville S, Hall A (2003) Cell polarity: Par6, aPKC and cytoskeletal crosstalk. Curr Opin Cell Biol 15: 67–72
Fidler IJ (2002) Critical determinants of metastasis. Semin Cancer Biol 12: 89–96
Ghosh S, Gifford AM, Riviere LR, Tempst P, Nolan GP, Baltimore D (1990) Cloning of the p50 DNA binding subunit of NF-kappa B: homology to rel and dorsal. Cell 62: 1019–1029
Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, Morrow M (2002) AJCC Cancer Staging Manual, TNM Classification of Malignant Tumors, 6th edn. Wiley-Liss, Springer-Verlag: New York
Guarino M (2007) Epithelial-mesenchymal transition and tumour invasion. Int J Biochem Cell Biol 39: 2153–2160
Hasegawa Y, Takanashi S, Kanehira Y, Tsushima T, Imai T, Okumura K (2001) Transforming growth factor-beta1 level correlates with angiogenesis, tumor progression, and prognosis in patients with nonsmall cell lung carcinoma. Cancer 91: 964–971
Havard L, Rahmouni S, Boniver J, Delvenne P (2005) High levels of p105 (NFKB1) and p100 (NFKB2) proteins in HPV16-transformed keratinocytes: role of E6 and E7 oncoproteins. Virology 331: 357–366
Hu L, Lau SH, Tzang CH, Wen JM, Wang W, Xie D, Huang M, Wang Y, Wu MC, Huang JF, Zeng WF, Sham JS, Yang M, Guan XY (2004) Association of Vimentin overexpression and hepatocellular carcinoma metastasis. Oncogene 23: 298–302
Ihde DC, Minna JD (1991) Non-small cell lung cancer. Part I: Biology, diagnosis, and staging. Curr Probl Cancer 15: 61–104
Ishikawa H, Claudio E, Dambach D, Raventos-Suarez C, Ryan C, Bravo R (1998) Chronic inflammation and susceptibility to bacterial infections in mice lacking the polypeptide (p)105 precursor (NF-kappaB1) but expressing p50. J Exp Med 187: 985–996
Janda E, Lehmann K, Killisch I, Jechlinger M, Herzig M, Downward J, Beug H, Grunert S (2002) Ras and TGF [beta] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J Cell Biol 156: 299–313
Jemal A, Thomas A, Murray T, Thun M (2002) Cancer statistics, 2002. CA Cancer J Clin 52: 23–47
Karin M, Cao Y, Greten FR, Li ZW (2002) NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2: 301–310
Kass L, Erler JT, Dembo M, Weaver VM (2007) Mammary epithelial cell: influence of extracellular matrix composition and organization during development and tumorigenesis. Int J Biochem Cell Biol 39: 1987–1994
Kureishy N, Sapountzi V, Prag S, Anilkumar N, Adams JC (2002) Fascins, and their roles in cell structure and function. Bioessays 24: 350–361
Leader M, Collins M, Patel J, Henry K (1987) Vimentin: an evaluation of its role as a tumour marker. Histopathology 11: 63–72
Liu D, Huang C, Kameyama K, Hayashi E, Yamauchi A, Kobayashi S, Yokomise H (2001) E-cadherin expression associated with differentiation and prognosis in patients with non-small cell lung cancer. Ann Thorac Surg 71: 949–954
Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL (2005) Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science 307: 1603–1609
Pelletier MP, Edwardes MD, Michel RP, Halwani F, Morin JE (2001) Prognostic markers in resectable non-small cell lung cancer: a multivariate analysis. Can J Surg 44: 180–188
Pelosi G, Pastorino U, Pasini F, Maissoneuve P, Fraggetta F, Iannucci A, Sonzogni A, De Manzoni G, Terzi A, Durante E, Bresaola E, Pezzella F, Viale G (2003) Independent prognostic value of fascin immunoreactivity in stage I nonsmall cell lung cancer. Br J Cancer 88: 537–547
Radisky DC, Bissell MJ (2007) NF-kappaB links oestrogen receptor signalling and EMT. Nat Cell Biol 9: 361–363
Regala RP, Weems C, Jamieson L, Khoor A, Edell ES, Lohse CM, Fields AP (2005) Atypical protein kinase C iota is an oncogene in human non-small cell lung cancer. Cancer Res 65: 8905–8911
Saji H, Nakamura H, Awut I, Kawasaki N, Hagiwara M, Ogata A, Hosaka M, Saijo T, Kato Y, Kato H (2003) Significance of expression of TGF-beta in pulmonary metastasis in non-small cell lung cancer tissues. Ann Thorac Cardiovasc Surg 9: 295–300
Thiery JP, Huang R (2005) Linking epithelial–mesenchymal transition to the well-known polarity protein Par6. Dev Cell 8: 456–458
Toloza EM, D’Amico TA (2005) Targeted therapy for non-small cell lung cancer. Semin Thorac Cardiovasc Surg 17: 199–204
Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC (2004) WHO classification of Tumours of the Lung, Pleura, Thymus and Heart. International Agency for Research on Cancer (IARC): Lyon., Ref Type: Generic
von Rahden BH, Stein HJ, Feith M, Puhringer F, Theisen J, Siewert JR, Sarbia M (2006) Overexpression of TGF-beta1 in esophageal (Barrett's) adenocarcinoma is associated with advanced stage of disease and poor prognosis. Mol Carcinog 45: 786–794
Weih F, Carrasco D, Bravo R (1994) Constitutive and inducible Rel/NF-kappa B activities in mouse thymus and spleen. Oncogene 9: 3289–3297
Zafrani B, Aubriot MH, Mouret E, De Cremoux P, De Rycke Y, Nicolas A, Boudou E, Vincent-Salomon A, Magdelenat H, Sastre-Garau X (2000) High sensitivity and specificity of immunohistochemistry for the detection of hormone receptors in breast carcinoma: comparison with biochemical determination in a prospective study of 793 cases. Histopathology 37: 536–545
Zhang Z, Ma J, Li N, Sun N, Wang C (2006) Expression of nuclear factor-kappaB and its clinical significance in nonsmall-cell lung cancer. Ann Thorac Surg 82: 243–248
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest
There are no financial or ethical conflicts of interest.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Al-Saad, S., Al-Shibli, K., Donnem, T. et al. The prognostic impact of NF-κB p105, vimentin, E-cadherin and Par6 expression in epithelial and stromal compartment in non-small-cell lung cancer. Br J Cancer 99, 1476–1483 (2008). https://doi.org/10.1038/sj.bjc.6604713
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6604713
Keywords
This article is cited by
-
LAMC2 promotes EGFR cell membrane localization and acts as a novel biomarker for tyrosine kinase inhibitors (TKIs) sensitivity in lung cancer
Cancer Gene Therapy (2023)
-
Unclear tumor border in magnetic resonance imaging as a prognostic factor of squamous cell cervical cancer
Scientific Reports (2023)
-
A thioredoxin reductase 1 inhibitor pyrano [3,2-a] phenazine inhibits A549 cells proliferation and migration through the induction of reactive oxygen species production
Molecular Biology Reports (2022)
-
PLK1/vimentin signaling facilitates immune escape by recruiting Smad2/3 to PD-L1 promoter in metastatic lung adenocarcinoma
Cell Death & Differentiation (2021)
-
Cell polarity and cell adhesion associated gene expression differences between invasive micropapillary and no special type breast carcinomas and their prognostic significance
Scientific Reports (2021)