Abstract
Paradoxical reactions (PRs) are poorly studied complex immunological phenomena, among patients with tuberculosis (TB). When PRs involves critical structures like the central nervous system (CNS), immunomodulatory therapy is often required. Predictors for PRs in TB to pre-empt appropriate treatment strategies in high-risk groups are lacking. TT genotype of Leukotriene A4 hydrolase (LTA4H) promoter region rs17525495 polymorphisms are associated with exaggerated immune responses in Tuberculous meningitis (TBM), the most severe form of extrapulmonary tuberculosis (EPTB). The association of these polymorphisms with PRs is not known. We evaluated this plausibility among 113 patients with EPTB, at high risk of PRs. Majority [81 (71.7%)] had disseminated tuberculosis with prominent CNS [54 (47.8%)] and lymph node involvement [47 (41.6%)]. Human immunodeficiency Virus (HIV) co-infection was seen among 23 (20.3%) patients. PRs were noted in 38.9% patients, at a median duration of 3 months (IQR 2–4). LTA4H rs17525495 single nucleotide polymorphism (SNP) analysis showed 52 (46%) patients had CC, 43 (38.1%) had CT and 18 (15.9%) had TT genotypes. There was no statistically significant difference in occurrence [CC 38.5% vs CT 39.5% vs TT 38.7%] and time of onset [median (IQR)] of PRs across the genotypes [CC 3 (1–4.7), CT 3 (2–5), TT 2 (2–3)]. PRs was shown to be significantly linked with HIV co-infection (RR 0.6, 95% CI 0.29–1.28), culture positivity (RR 0.5, 95% CI 0.28–1.14), TB Lymphadenitis (RR 0.7, 95% CI 0.44–1.19) and CNS involvement RR 2.1, 95% CI 1.27–3.49) in the univariate analysis (p < 0.2). On multivariate analysis, CNS involvement alone was associated with PRs (aRR 3.8 (1.38–10.92); p < 0.01). PRs were associated with CNS involvement but not with LTA4H rs17525495 polymorphisms.
Similar content being viewed by others
Introduction
Paradoxical reaction refers to worsening of pre-existing tubercular lesions or appearance of new lesions after initial clinical improvement while on appropriate antitubercular therapy (ATT). It complicates up to a third of patients with EPTB and remains poorly studied1. The underlying mechanism appears to be immunologically mediated and variable in timing of occurrence, duration and severity. Most patients with PRs involving critical areas like the CNS require immunomodulatory therapy like steroids and upfront identification of these patients is important. The unpredictable nature of the PRs makes clinicians suspect drug resistance, therapy failure and alternative diagnosis. This makes PRs difficult to study and it remains an under researched area. Upfront identification of these patients can help clinicians to optimise therapy avoiding further diagnostic evaluation. Reported risk factors in the literature include younger age, female gender, shorter duration of symptoms, HIV co-infection, lower blood lymphocyte count, disseminated disease, acid fast bacilli positivity on smear and worsening of cerebrospinal fluid (CSF) parameters among patients with CNS tuberculosis2,3. However, most of these risk factors are non-specific. Recently, a SNP in the promoter region of the LTA4H rs17525495 (TT) was shown to predict hyperinflammatory cytokine phenotype and response to immunomodulation in TBM survival and severe immune reconstitution and inflammatory syndrome in HIV associated pulmonary TB4,5,6. There is biological plausibility for this association as LTA4H is a key enzyme in eicosanoid synthesis modulating the balance between its products—pro-inflammatory Leukotriene B4 (LTB4) and anti-inflammatory Lipoxin A4 (LXA4)7. We hypothesised that hyper inflammatory genotype of the LTA4H polymorphism (TT) may be associated with PRs. The incidence of PRs is not equally distributed among patients with EPTB. Patients with disseminated disease, CNS and nodal involvement are particularly high risk for PRs, providing enriched group to study this hypothesis. This may provide the first genomic test to predict PRs, identifying a group of patients who require pre-emptive immunomodulatory treatment.
Results
Our study population included 113 adults, predominantly males (54.9%) from South (44.2%) and East India (55.8%) (Table 1). The mean age of our patients was 33.8 ± 11.7 years. One in five (20.4%) patients had HIV co-infection. Most of the study population (73.4%) had microbiologically confirmed TB. Rest of the patients had clinical features of TB supported by either histopathological or radiological evidence. Eleven patients had resistant TB (5 had multidrug resistant TB, 5 had isoniazid mono-resistance, 1 had Pyrazinamide mono-resistance). Disseminated TB (71.7%) was most common, followed by CNS TB (47.8%) and TB lymphadenitis (41.6%). In those with TBM (n = 54), 77.5% fulfilled Lancet consensus scoring criteria for definite or probable TBM. Eight (14.8%) had Medical Research Council (MRC) grade-3 disease of TBM.
More than one third (38.9%) of our patients developed PRs at 3 months (IQR 2–4) after initiating ATT. PRs resulted in modification of therapy in 28 (63.6%) patients in the form of modification of steroid dose or administration of immunomodulators other than steroids. No patient had change in ATT in view of PRs. Overall, CC genotype (46%) was found more common than CT (38.1%) and TT (15.9%) genotypes. In the univariate analysis, HIV co-infection (RR 0.6, 95% CI 0.29–1.28), culture positivity (RR 0.5, 95% CI 0.28–1.14), TB lymphadenitis (RR 0.7, 95% CI 0.44–1.19) and CNS involvement (RR 2.1, 95% CI 1.27–3.49) were found to be significantly associated with PRs. However, when these predictors were taken into multivariate model, only CNS involvement had almost fourfold [aRR (95% CI), 3.8 (1.38–10.92), P = 0.010] higher risk of developing PRs than their counterparts.
Of the 23 patients with HIV, 19 had Xpert positive results, of which 5 (26.3%) showed PR while 14 (73.7%) did not show PR. Among the four patients with Xpert negative results, only 1 (25%) showed PR while 3 (75%) did not show PR. This finding was found statistically insignificant with p = 1.000. Eight percent of patients were non-adherent to the ATT treatment where 44.4% of them showed PR and 55.6% did not show PR (p = 0.734).
We also analysed the distribution of LTA4H rs17525495 polymorphism genotypes (CC, CT, TT) with the baseline characteristics and outcomes (Table 2). We found there was no significant association between baseline characters and genotypes including culture positivity (CC 23.1% vs CT 30.2% vs TT 16.7%) and severity of TBM. We noted no statistically significant association in the occurrence (CC 38.5% vs CT 39.5% vs TT 38.7%) or time of onset [median (IQR)] [CC 3 (1–4.7), CT 3 (2–5), TT 2 (2–3)] of PRs and the genotypes. Three patients out of 113 died, two had CNS TB with CC genotype and one with CT genotype.
Discussion
PR often complicates patients with EPTB on ATT. In our study focussing on patients at high risk of PR, its prevalence was 38.9% and LTA4H rs17525495 polymorphisms were not related to their occurrence. TT genotype of LTA4H rs17525495 polymorphism is linked to hyperinflammation and the benefit of immunomodulatory therapy in CNS TB. This is the first study globally to evaluate whether these genotypes are associated with PRs. PRs are compartmentalised immunological phenomena, often without systemic symptoms. Hypothetically, these may be driven by complex interplay between the pathogen and host response in immunologically privileged areas like CNS, while LTA4H rs17525495 genotypes may have a broad role modulating immune responses at the organism level.
Close to two thirds (63.6%) of patients with PRs required immunomodulatory therapy. This is reflective of the large proportion of patients with CNS involvement in the study. While PRs can be self -limiting in milder forms of EPTB, it can be severe requiring therapy modification in others. CNS involvement alone was found to be high risk for the development of PRs. This observation may have implications—biomarkers in the blood may not reflect these immunological changes and evaluation of representative samples are needed, as reported by others8. Despite increased occurrence of PRs among people living with HIV (84.6%), it did not reach statistical significance, probably due to type II error9.
While the global distribution of rs17525495 SNP genotypes is similar across the Vietnamese, Indonesian, Zambian and our study cohorts, important differences exist in the in vivo and in vitro observations5,10,11. Zebrafish larvae modelling studies show the LTA4H gene regulates inflammatory milieu by controlling the balance between pro- and anti-inflammatory eicosanoids and Tumor necrosis factor-alpha (TNF-α) production. The homozygous genotypes (CC and TT) are associated with accelerated bacterial growth in the larval stages while the heterozygotes (CT) are protected from severe Mycobacterium marinum infection7. However, the picture is more complex in humans. In large TBM cohorts, heterozygosity (CT) corresponds to balanced inflammatory response whereas homozygosity (CC/TT) represents polar spectrums of disease. CC correlates to hypo-inflammation with low TNF alpha levels and increased bacterial burden while TT with high TNF alpha levels, controlled mycobacterial burden and hyperinflammatory host immune response5. TT genotype predicted high pro-inflammatory cytokine milieu in the CSF and the response to corticosteroids among HIV uninfected patients in Vietnam but not in Indonesia and Zambia5,10,11. Among HIV-pulmonary TB coinfected patients, TT genotype was associated with severe IRIS phenomena6. Similar association was not reported in the Vietnamese cohort with CNS TB5.
Our study cohort has important differences from those published in the literature. Few studies have evaluated PR in many types of EPTB3. We had high rates of disseminated disease, CNS involvement and patients requiring immunomodulation as we preferentially recruited patients at high risk of PR. The rates of PR in our study were similar to TBM cohorts but lesser than cohorts with several types of EPTB2,9. The median duration of PR in our study was similar to TBM cohorts but longer than other EPTB data3,9.
What could explain such apparent diverse observations? Firstly, PRs may be caused by local immune responses in immune sanctuary sites like CNS and are not controlled by host genotypes. This is also supported by paucity of systemic response like fever or elevated inflammatory markers in non-HIV infected patients with PRs. Though majority of our patients had disseminated tuberculosis, the PRs are often localised to a single organ2,3,9. Alternatively, the effect of LTA4H polymorphisms may be modulated by other gene systems and epigenetic factors in various populations. Studies reporting on LTA4H polymorphisms could consider measuring the gene products while evaluating their association with clinical outcomes. Thirdly, significant variability across regions may be due to population genetic factors like “Linkage Disequilibrium”12. Linkage disequilibrium is the non-random association of alleles at different loci in diverse populations. Within ethnic groups, certain genetic loci or alleles of interest are conserved and remain unchanged in a particular position. This may lead to erroneous implication of a tested SNP allele as the cause of a phenotype when, a second unknown SNP with alleles strongly correlated with the tested one is actually responsible. This may explain the difference in association of LTA4H SNPs with TB survival in the Indonesian and Vietnamese population13,14. Our study shows TT genotype had a trend towards lesser culture positivity (16.7%) compared to hypo-inflammatory CC type (23.1%), suggesting the potential role of hyper-inflammatory TT genotype in restricting mycobacterial replication, probably due to excessive inflammation (Table 2). Similar findings were also observed in the Zambian study10.
Our study has several limitations. While the sample size was small, a significant number of patients developed PRs. It is a single centre study and the distribution of the genotypes of our cohort represents only a restricted topography. Since our study focussed on CNS and lymph nodal TB, the findings may not be applicable for all EPTB. In summary, PRs were associated with CNS involvement but not LTA4H rs17525495 polymorphisms in our study.
Methods and material
We conducted a retrospective cohort study among adult patients with EPTB, at high risk of PR, in the Departments of Infectious Diseases and Neurological Sciences at Christian Medical College and Hospital, Vellore, India from 1st June, 2020 to 31st December, 2021. We recruited patients with disseminated, CNS and nodal involvement preferentially in view of higher incidence and clinical impact in these groups. We retrieved patient's clinical characteristics, laboratory findings, Mycobacterium tuberculosis genotypic and phenotypic resistance, treatment regimens, LTA4H genotypic status, imaging data and clinical or radiological evidence of PRs from the hospital electronic database. Tuberculosis in the study was defined as follows: patients with clinical features of TB with one or more of the following—(1) Microbiological evidence (mycobacterial culture positivity or Cepheid GeneXpert positive for Mycobacterium tuberculosis), (2) Histopathology showing necrotizing granulomatous inflammation, (3) Radiological evidence supporting TB (where tissue diagnosis is not feasible e.g. CNS TB) were included in the study. We defined disseminated tuberculosis as having two or more non-contiguous sites15. Patients who did not develop PRs were considered as controls. All the patients included in the study completed 3 months of follow-up, correlating with the period of PRs occurrence. During each visit, clinical improvement, PRs, weight of the patient and compliance on ATT were documented. Dose of ATT was optimised as per the weight of the patient at each visit. Non-adherence was defined as patients missing ≥ 10% of total prescribed dose16. ‘Modification of therapy’ in the study refers to one of the three after the onset of PRs—(1) Increase in the dose of steroids (2) Addition of steroids, or (3) Administration of immunomodulators other than steroids in addition to weight based anti-tuberculous therapy. The International Network for the Study of HIV-associated Immune reconstitution inflammatory syndrome [IRIS] (INSHI) consensus case definition was used to define PRs17. It requires a diagnosis of TB with an initial positive response to treatment, characteristic clinical features (such as new or enlarging lymphadenopathy, constitutional, respiratory or abdominal symptoms, or new or worsening radiological features), and exclusion of alternative explanations for clinical deterioration. Patients with TBM were classified as definite, probable and possible as per the Lancet Consensus scoring system for TBM diagnosis18. The management of PRs were decided by the respective treating physicians.
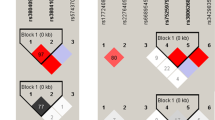
Genetic analysis for LTA4H rs17525495 polymorphisms were done as a part of routine care in our patients in line with the emerging association with outcomes in severe forms of tuberculosis9. Genomic DNA from the samples was isolated using Qiagen Mini DNA blood kit. The genotyping of SNP rs17525495 in these samples were done by a PCR–RFLP method developed by our Neurochemistry Laboratory and was validated previously using a commercially available TaqMan assay kit and Sanger sequencing. PCR, which yielded a product of 175 bp, was performed for 30 cycles using the primers 5′-TTAAGAAACTTCCTTTCCCGGC-3′ and 5′-CCAACGAACAGGTATCCACTAT-3′ with 58 °C as annealing temperature. The PCR products were digested with the restriction enzyme HpyAV (2.5 units/sample) for 6 h at 37 °C and were resolved in 2% agarose gel after digestion. As this enzyme cuts the sample with T allele into two fragments, we identified the CC genotype by the presence of an uncut 175 bp fragment, CT genotype by 175, 103 and 72 bp fragments and TT genotype by 103 and 72 bp fragments.
We also evaluated the association of PRs with multiple clinical parameters like organ involvement, presence of disseminated disease, mycobacterial culture positivity, HIV co-infection, drug resistant tuberculosis and suboptimal TB treatment.
The descriptive statistics of socio-demographic and other clinical parameters of the study population were analysed using frequency and percentages for categorical variables; mean and standard deviation (SD), median and inter quartile range (IQR) for continuous variables. Associations between PRs and clinical predictors were assessed using chi-square tests or Fisher’s exact tests as appropriate. An independent sample t-test was performed to find the mean difference between two groups with continuous variables. Factors with a p < 0.20 were considered for inclusion in the multivariate analysis. Multivariate Logistic Regression analysis was performed to assess the independent effects of factors associated with PRs. To account for the multiplicity of tests, a Bonferroni correction was applied taking a two-sided P-value of 0.01 as significance level. All analyses were performed using the software programs SPSS (version 23).
Ethics declaration and consent to participate
Ethical approval was obtained from the Institutional Review Board (IRB Min No. 14456) of Christian Medical College and hospital, Vellore, Tamil Nadu, India. The IRB waived participant consent as this is a retrospective study. Additionally, all methods were performed in accordance with the relevant guidelines and regulations.
Data availability
Data are available upon reasonable request to the corresponding author.
Abbreviations
- LTA4H :
-
Leukotriene A4 hydrolase
- PRs:
-
Paradoxical reactions
- TBM:
-
Tuberculous meningitis
- EPTB:
-
Extrapulmonary tuberculosis
- TB:
-
Tuberculosis
- CNS:
-
Central nervous system
- HIV:
-
Human immunodeficiency virus
- ATT:
-
Anti tuberculosis therapy
- IQR:
-
Interquartile range
- CSF:
-
Cerebrospinal fluid
- SNP:
-
Single nucleotide polymorphism
- MRC grading:
-
Medical Council of Research grading system for tuberculous meningitis
- TNF-α:
-
Tumor necrosis factor-alpha
- IRIS:
-
Immune reconstitution inflammatory syndrome
- INSHI:
-
International Network for the Study of HIV-associated Immune IRIS
- SD:
-
Standard deviation
- SC:
-
Spinal cord
References
Geri, G. et al. Paradoxical reactions during treatment of tuberculosis with extrapulmonary manifestations in HIV-negative patients. Infection 41(2), 537–543 (2013).
Lu, Y. et al. Worsening CSF parameters after the start of anti-tuberculosis treatment predicts intracerebral tuberculoma development. Int. J. Infect. Dis. 1(101), 395–402 (2020).
Barr, D. A. et al. Paradoxical upgrading reaction in extra-pulmonary tuberculosis: Association with vitamin D therapy. Int. J. Tuberc. Lung Dis. 21(6), 677–683. https://doi.org/10.5588/ijtld.16.0927 (2017).
Whitworth, L. et al. A Bayesian analysis of the association between Leukotriene A4 Hydrolase genotype and survival in tuberculous meningitis. Elife 10, e61722. https://doi.org/10.7554/eLife.61722 (2021).
Thuong, N. T. T. et al. Leukotriene A4 hydrolase genotype and HIV infection influence intracerebral inflammation and survival from tuberculous meningitis. J. Infect. Dis. 215(7), 1020–1028 (2017).
Narendran, G. et al. Role of LTA4H polymorphism in tuberculosis-associated immune reconstitution inflammatory syndrome occurrence and clinical severity in patients infected with HIV. PLoS ONE 11(9), e0163298 (2016).
Tobin, D. M. et al. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell 148(3), 434–446 (2012).
Rohlwink, U. K. et al. Biomarkers of cerebral injury and inflammation in pediatric tuberculous meningitis. Clin. Infect. Dis. 65(8), 1298–1307. https://doi.org/10.1093/cid/cix540 (2017).
Singh, A. K. et al. Paradoxical reaction in tuberculous meningitis: Presentation, predictors and impact on prognosis. BMC Infect. Dis. 16, 306. https://doi.org/10.1186/s12879-016-1625-9 (2016).
Van Laarhoven, A. et al. Clinical parameters, routine inflammatory markers, and LTA4H genotype as predictors of mortality among 608 patients with tuberculous meningitis in Indonesia. J. Infect. Dis. 215(7), 1029–1039 (2017).
Siddiqi, O. K. et al. LTA4H prevalence and mortality in adult Zambians with tuberculous meningitis. Ann. Neurol. 90(6), 994–998 (2021).
Tobin, D. M. et al. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell 140(5), 717–730 (2010).
Fava, V. M. et al. Evaluating the impact of LTA4H genotype and immune status on survival from tuberculous meningitis. J. Infect. Dis. 215(7), 1011–1013 (2017).
Slatkin, M. Linkage disequilibrium-understanding the evolutionary past and mapping the medical future. Nat. Rev. Genet. 9(6), 477–485. https://doi.org/10.1038/nrg2361 (2008).
Ayaslioglu, E. et al. Disseminated tuberculosis with lymphatic, splenic and scrotal abscesses: A case report. Cases J. 2, 6995. https://doi.org/10.4076/1757-1626-2-6995 (2009).
Mekonnen, H. S. & Azagew, A. W. Non-adherence to anti-tuberculosis treatment, reasons and associated factors among TB patients attending at Gondar town health centers, Northwest Ethiopia. BMC Res. Notes. 11(1), 691. https://doi.org/10.1186/s13104-018-3789-4 (2018).
Meintjes, G. et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: Case definitions for use in resource-limited settings. Lancet. Infect. Dis 8(8), 516–523 (2008).
Sulaiman, T. et al. The diagnostic utility of the “Thwaites’ system” and “lancet consensus scoring system” in tuberculous vs non-tuberculous subacute and chronic meningitis: Multicentre analysis of 395 adult patients. BMC Infect. Dis. 20(1), 788 (2020).
Acknowledgements
The authors gratefully acknowledge Dr Abraham Hosea Lamech, MBBS and Mr Silas K for their technical support.
Funding
The research received no specific grant from any funding agency, commercial or non-for-profit sectors.
Author information
Authors and Affiliations
Contributions
S.S.K., A.M. conceptualised the study; S.S.K., P.G. collected the data; P.G., did the primary analysis of the data; S.S.K., A.M. wrote the original draft; R.S., C.S.C. defined & wrote the methodology for the laboratory analysis of samples in the study; P.S. validated the statistical analysis; S.S.K., P.G., L.R.I, A.S., J.S.M., R.K., G.M.V, C.S.C. and A.M. critically reviewed and validated the final version of the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kumar, S.S., Solomon, R., Gautam, P. et al. Leukotriene A4 hydrolase (LTA4H rs17525495) gene polymorphisms and paradoxical reactions in extrapulmonary tuberculosis. Sci Rep 13, 3746 (2023). https://doi.org/10.1038/s41598-023-30923-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30923-2
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.