Abstract
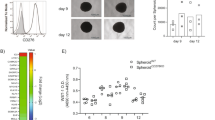
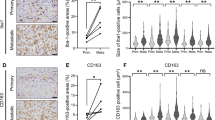
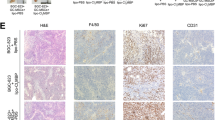
Tumor-associated macrophages (TAMs) have multifaceted roles in tumor development, particularly linked with tumor angiogenesis and invasion, but the molecular mechanism underlying this association remains unclear. In this study, we report that lack of osteopontin (OPN) suppresses melanoma growth in opn−/− mice and macrophages are the crucial component responsible for OPN-regulated melanoma growth. In tumor microenvironment, OPN activates macrophages and influences angiogenesis by enhancing cyclooxygenase-2 (COX-2)-dependent prostaglandin E2 (PGE2) production in an autocrine manner. Furthermore, we identify α9β1 integrin as a functional receptor for OPN that mediates its effect and activates ERK and p38 signaling, which ultimately leads to COX-2 expression in macrophages. The major role played by OPN and PGE2 in angiogenesis are further amplified by upregulation of MMP-9. OPN-activated macrophages promote the migration of endothelial and cancer cells via PGE2. These findings provide evidence that TAMs serve as source of key components such as OPN and COX-2-derived PGE2 and MMP-9 in melanoma microenvironment. Clinical specimens analyses revealed that increased infiltration of OPN-positive TAMs correlate with melanoma growth and angiogenesis. These data provide compelling evidence that OPN and COX-2 expressing macrophages are obligatory factors in melanoma growth. We conclude that OPN signaling is involved in macrophage recruitment into tumor, and our results emphasize the potential role of macrophage in modulation of tumor microenvironment via secretion of OPN, PGE2 and MMP-9, which trigger angiogenesis and melanoma growth. Thus, blockade of OPN and its regulated signaling network provides unique strategy to eradicate melanoma by manipulating TAMs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Lazar-Molnar E, Hegyesi H, Toth S, Falus A . Autocrine and paracrine regulation by cytokines and growth factors in melanoma. Cytokine 2000; 12: 547–554.
Nesbit M, Schaider H, Miller TH, Herlyn M . Low-level monocyte chemoattractant protein-1 stimulation of monocytes leads to tumor formation in nontumorigenic melanoma cells. J Immunol 2001; 166: 6483–6490.
Bhowmick NA, Moses HL . Tumor-stroma interactions. Curr Opin Genet Dev 2005; 15: 97–101.
Mueller MM, Fusenig NE . Friends or foes-bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 2004; 4: 839–849.
Vosseler S, Mirancea N, Bohlen P, Mueller MM, Fusenig NE . Angiogenesis inhibition by vascular endothelial growth factor receptor-2 blockade reduces stromal matrix metalloproteinase expression, normalizes stromal tissue and reverts epithelial tumor phenotype in surface heterotransplants. Cancer Res 2005; 65: 1294–1305.
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A . Macrophage polarization: tumor- associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002; 23: 549–555.
Gazzaniga S, Bravo AI, Guglielmotti A, van Rooijen N, Maschi F, Vecchi A et al. Targeting tumor-associated macrophages and inhibition of MCP-1 reduce angiogenesis and tumor growth in a human melanoma xenograft. J Invest Dermatol 2007; 127: 2031–2041.
Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res 2006; 66: 11238–11246.
Denhardt DT, Guo X . Osteopontin: a protein with diverse functions. FASEB J 1993; 7: 1475–1482.
Rangaswami H, Bulbule A, Kundu GC . Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol 2006; 16: 79–87.
Weber GF, Ashkar S, Glimcher MJ, Cantor H . Receptor-ligand interaction between CD44 and osteopontin (Eta-1). Science 1996; 271: 509–512.
Panda D, Kundu GC, Lee BI, Peri A, Fohl D, Chackalaparampil I et al. Potential roles of osteopontin and alphaVbeta3 integrin in the development of coronary artery restenosis after angioplasty. Proc Natl Acad Sci USA 1997; 94: 9308–9313.
Jain S, Chakraborty G, Kundu GC . The crucial role of cyclooxygenase-2 in osteopontin-induced protein kinase C alpha/c-Src/IkappaB kinase alpha/beta dependent prostate tumor progression and angiogenesis. Cancer Res 2006; 66: 6638–6648.
Behera R, Kumar V, Lohite K, Karnik S, Kundu GC . Activation of JAK2/STAT3 signaling by osteopontin promotes tumor growth in human breast cancer cells. Carcinogenesis 2010; 31: 192–200.
Chakraborty G, Jain S, Kundu GC . Osteopontin promotes vascular endothelial growth factor-dependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res 2008; 68: 152–161.
Ahmed M, Kundu GC . Osteopontin selectively regulates p70S6K/mTOR phosphorylation leading to NF-κB dependent AP-1-mediated ICAM-1 expression in breast cancer cells. Mol Cancer 2010; 9: 101.
Nakao S, Kuwano T, Tsutsumi-Miyahara C, Ueda S, Kimura YN, Hamano S et al. Infiltration of COX-2-expressing macrophages is a prerequisite for IL-1β-induced neovascularization and tumor growth. J Clin Invest 2005; 115: 2979–2991.
Bianchini F, Massi D, Marconi C, Franchi A, Baroni G, Santucci M et al. Expression of cyclo-oxygenase-2 in macrophages associated with cutaneous melanoma at different stages of progression. Prostaglandins other Lipid Mediat 2007; 83: 320–328.
Finetti F, Donnini S, Giachetti A, Morbidelli L, Ziche M . Prostaglandin E2 primes the angiogenic switch via a synergic interaction with the fibroblast growth factor-2 pathway. Circ Res 2009; 105: 657–666.
Baxevanis CN, Reclos GJ, Gritzapis AD, Dedousis GV, Missitzis I, Papamichail M . Elevated prostaglandin E2 production by monocytes is responsible for the depressed levels of natural killer and lymphokine-activated killer cell function in patients with breast cancer. Cancer 1993; 72: 491–501.
Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL . Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 1996; 56: 4625–4629.
Fujimoto J, Sakaguchi H, Aoki I, Tamaya T . Clinical implications of expression of interleukin 8 related to angiogenesis in uterine cervical cancers. Cancer Res 2000; 60: 2632–2635.
Torisu H, Ono M, Kiryu H, Furue M, Ohmoto Y, Nakayama J et al. Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanoma: possible involvement of TNF alpha and IL-1alpha. Int J Cancer 2000; 85: 182–188.
Nishie A, Ono M, Shono T, Fukushi J, Otsubo M, Onoue H et al. Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin Cancer Res 1999; 5: 1107–1113.
Salvesen HB, Akslen LA . Significance of tumour-associated macrophages, vascular endothelial growth factor and thrombospondin-1 expression for tumour angiogenesis and prognosis in endometrial carcinomas. Int J Cancer 1999; 84: 538–543.
Lissbrant IF, Stattin P, Wikstrom P, Damber JE, Egevad L, Bergh A . Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. Int J Oncol 2000; 17: 445–451.
Nemoto H, Rittling SR, Yoshitake H, Furuya K, Amagasa T, Tsuji K et al. Osteopontin deficiency reduces experimental tumor cell metastasis to bone and soft tissues. J Bone Miner Res 2001; 16: 652–659.
Ojalvo LS, King W, Cox D, Pollard JW . High-density gene expression analysis of tumor-associated macrophages from mouse mammary tumors. Am J Pathol 2009; 174: 1048–1064.
Shin HS, Jung CH, Park HD, Lee SS . The relationship between the serum intercellular adhesion molecule-1 level and the prognosis of the disease in lung cancer. Korean J Intern Med 2004; 19: 48–52.
Naylor MS, Stamp GW, Davies BD, Balkwill FR . Expression and activity of MMPS and their regulators in ovarian cancer. Int J Cancer 1994; 58: 50–56.
Nikkola J, Vihinen P, Vuoristo MS, Kellokumpu-Lehtinen P, Kähäri VM, Pyrhönen S . High serum levels of matrix metalloproteinase-9 and matrix metalloproteinase-1 are associated with rapid progression in patients with metastatic melanoma. Clin Cancer Res 2005; 11: 5158–5166.
Giraudo E, Inoue M, Hanahan D . An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Inves 2004; 114: 623–633.
Bachmann IM, Ladstein RG, Straume O, Naumov GN, Akslen LA . Tumor necrosis is associated with increased alphavbeta3 integrin expression and poor prognosis in nodular cutaneous melanomas. BMC Cancer 2008; 8: 362.
Crawford HC, Matrisian LM, Liaw L . Distinct roles of osteopontin in host defense activity and tumor survival during squamous cell carcinoma progression in vivo. Cancer Res 1998; 58: 5206–5215.
Feng B, Rollo EE, Denhardt DT . Osteopontin (OPN) may facilitate metastasis by protecting cells from macrophage NO-mediated cytotoxicity: evidence from cell lines downregulated for OPN expression by a targeted ribozyme. Clin Exp Metastasis 1995; 13: 453–462.
Gorelik E, Wiltrout RH, Brunda MJ, Holden HT, Herberman RB . Augmentation of metastasis formation by thioglycollate-elicited macrophages. Int J Cancer 1982; 29: 575–581.
Rhodes DR, Kalyana-Sundaram S, Mahavisno V, Varambally R, Yu J, Briggs BB et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia 2007; 9: 166–180.
Hsu HP, Shan YS, Lai MD, Lin PW . Osteopontin-positive infiltrating tumor-associated macrophages in bulky ampullary cancer predict survival. Cancer Biol Ther 2010; 10: 144–154.
Liguori M, Solinas G, Germano G, Mantovani A, Allavena P . Tumor-associated macrophages as incessant builders and destroyers of the cancer stroma. Cancers 2011; 3: 3740–3761.
Brown LF, Papadopoulos-Sergiou A, Berse B, Manseau EJ, Tognazzi K, Perruzzi CA et al. Osteopontin expression and distribution in human carcinomas. Am J Pathol 1994; 145: 610–623.
Solinas G, Schiarea S, Liguori M, Fabbri M, Pesce S, Zammataro L et al. Tumor-conditioned macrophages secrete migration-stimulating factor: a new marker for M2-polarization, influencing tumor cell motility. J Immunol 2010; 185: 642–652.
Cheng J, Huo DH, Kuang DM, Yang J, Zheng L, Zhuang SM . Human macrophages promote the motility and invasiveness of osteopontin-knockdown tumor cells. Cancer Res 2007; 67: 5141–5147.
Lewis CE, Leek R, Harris A, McGee JO . Cytokine regulation of angiogenesis in breast cancer: the role of tumor-associated macrophages. J Leukoc Biol 1995; 57: 747–751.
Nakanishi Y, Nakatsuji M, Seno H, Ishizu S, Akitake-Kawano R, Kanda K et al. COX-2 inhibition alters the phenotype of tumor-associated macrophages from M2 to M1 in ApcMin/+mouse polyps. Carcinogenesis 2011; 32: 1333–1339.
Heusinkveld M, de Vos van Steenwijk PJ, Goedemans R, Ramwadhdoebe TH, Gorter A, Welters MJ et al. M2 macrophages induced by prostaglandin E2 and IL-6 from cervical carcinoma are switched to activated M1 macrophages by CD4+ Th1 cells. J Immunol 2011; 187: 1157–1165.
Green CE, Liu T, Montel V, Hsiao G, Lester RD, Subramaniam S et al. Chemoattractant signaling between tumor cells and macrophages regulates cancer cell migration, metastasis and neovascularization. PLoS One 2009; 4: e6713.
Wang S, Raven JF, Baltzis D, Kazemi S, Brunet DV, Hatzoglou M et al. The catalytic activity of the eukaryotic initiation factor-2α kinase PKR is required to negatively regulate Stat1 and Stat3 via activation of the T-cell protein-tyrosine phosphatase. J Biol Chem 2006; 281: 9439–9449.
Kumar V, Behera R, Lohite K, Karnik S, Kundu GC . p38 kinase is crucial for osteopontin-induced furin expression that supports cervical cancer progression. Cancer Res 2010; 70: 10381–10391.
Sharma P, Kumar S, Kundu GC . Transcriptional regulation of human osteopontin promoter by histone deacetylase inhibitor, trichostatin A in cervical cancer cells. Mol Cancer 2010; 9: 178.
Chakraborty G, Jain S, Kale S, Raja R, Kumar S, Mishra R et al. Curcumin suppresses breast tumor angiogenesis by abrogating osteopontin-induced VEGF expression. Mol Med Rep 2008; 1: 641–646.
Rangaswami H, Bulbule A, Kundu GC . Nuclear factor-inducing kinase plays a crucial role in osteopontin-induced MAPK/IkappaBalpha kinase-dependent nuclear factor kappaB-mediated promatrix metalloproteinase-9 activation. J Biol Chem 2004; 279: 38921–38935.
Jain S, Chakraborty G, Raja R, Kale S, Kundu GC . Prostaglandin E2 regulates tumor angiogenesis in prostate cancer. Cancer Res 2008; 68: 7750–7759.
Acknowledgements
We thank Dr B Ramanamurthy, In-charge, Experimental Animal Facility, National Center for Cell Science, Pune, India for breeding and maintaining the Opn−/− mice. We also thank Dr S Ghaskabdi and Dr K Pai, Department of Zoology, University of Pune, India for providing the facility for CAM assay. Skillful assistance was provided by Dr S Vishwakarma, Department of Pathology, YCM Hospital, Pune, India for preparation of Cryosections. The project was supported by Department of Biotechnology (DBT) (GCK), Council of Scientific and Industrial Research (CSIR) (SK and GS), Indian Council of Medical Research (ICMR) (RR) and Department of Science and Technology (DST) (DT), Government of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Kale, S., Raja, R., Thorat, D. et al. Osteopontin signaling upregulates cyclooxygenase-2 expression in tumor-associated macrophages leading to enhanced angiogenesis and melanoma growth via α9β1 integrin. Oncogene 33, 2295–2306 (2014). https://doi.org/10.1038/onc.2013.184
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.184
Keywords
This article is cited by
-
SPP1+ TAM subpopulations in tumor microenvironment promote intravasation and metastasis of head and neck squamous cell carcinoma
Cancer Gene Therapy (2024)
-
Macrophage’s role in solid tumors: two edges of a sword
Cancer Cell International (2023)
-
Tumor-associated macrophage derived IL-6 enriches cancer stem cell population and promotes breast tumor progression via Stat-3 pathway
Cancer Cell International (2022)
-
Tumor-derived osteopontin drives the resident fibroblast to myofibroblast differentiation through Twist1 to promote breast cancer progression
Oncogene (2021)
-
The histone lysine methyltransferase SETD8 regulates angiogenesis through HES-1 in human umbilical vein endothelial cells
Scientific Reports (2020)