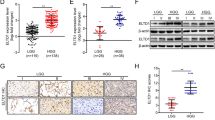
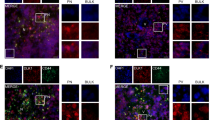
Abstract
Sustaining a high growth rate requires tumors to exploit resources in their microenvironment. One example of this is the extensive angiogenesis that is a typical feature of high-grade gliomas. Here, we show that expression of the constitutively active mutant epidermal growth factor receptor, ΔEGFR (EGFRvIII, EGFR*, de2-7EGFR) is associated with significantly higher expression levels of the pro-angiogenic factor interleukin (IL)-8 in human glioma specimens and glioma stem cells. Furthermore, the ectopic expression of ΔEGFR in different glioma cell lines caused up to 60-fold increases in the secretion of IL-8. Xenografts of these cells exhibit increased neovascularization, which is not elicited by cells overexpressing wild-type (wt)EGFR or ΔEGFR with an additional kinase domain mutation. Analysis of the regulation of IL-8 by site-directed mutagenesis of its promoter showed that ΔEGFR regulates its expression through the transcription factors nuclear factor (NF)-κB, activator protein 1 (AP-1) and CCAAT/enhancer binding protein (C/EBP). Glioma cells overexpressing ΔEGFR showed constitutive activation and DNA binding of NF-κB, overexpression of c-Jun and activation of its upstream kinase c-Jun N-terminal kinase (JNK) and overexpression of C/EBPβ. Selective pharmacological or genetic targeting of the NF-κB or AP-1 pathways efficiently blocked promoter activity and secretion of IL-8. Moreover, RNA interference-mediated knock-down of either IL-8 or the NF-κB subunit p65, in ΔEGFR-expressing cells attenuated their ability to form tumors and to induce angiogenesis when injected subcutaneously into nude mice. On the contrary, the overexpression of IL-8 in glioma cells lacking ΔEGFR potently enhanced their tumorigenicity and produced highly vascularized tumors, suggesting the importance of this cytokine and its transcription regulators in promoting glioma angiogenesis and tumor growth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Antonyak MA, Moscatello DK, Wong AJ . (1998). Constitutive activation of c-Jun N-terminal kinase by a mutant epidermal growth factor receptor. J Biol Chem 273: 2817–2822.
Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ et al. (2002). Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell 1: 269–277.
Bancroft CC, Chen Z, Yeh J, Sunwoo JB, Yeh NT, Jackson S et al. (2002). Effects of pharmacologic antagonists of epidermal growth factor receptor, PI3K and MEK signal kinases on NF-kappaB and AP-1 activation and IL-8 and VEGF expression in human head and neck squamous cell carcinoma lines. Int J Cancer 99: 538–548.
Biernat W, Huang H, Yokoo H, Kleihues P, Ohgaki H . (2004). Predominant expression of mutant EGFR (EGFRvIII) is rare in primary glioblastomas. Brain Pathol 14: 131–136.
Biswas DK, Cruz AP, Gansberger E, Pardee AB . (2000). Epidermal growth factor-induced nuclear factor kappa B activation: a major pathway of cell-cycle progression in estrogen-receptor negative breast cancer cells. Proc Natl Acad Sci USA 97: 8542–8547.
Brat DJ, Bellail AC, Van Meir EG . (2005). The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol 7: 122–133.
Brew R, Erikson JS, West DC, Kinsella AR, Slavin J, Christmas SE . (2000). Interleukin-8 as an autocrine growth factor for human colon carcinoma cells in vitro. Cytokine 12: 78–85.
Brown PH, Alani R, Preis LH, Szabo E, Birrer MJ . (1993). Suppression of oncogene-induced transformation by a deletion mutant of c-jun. Oncogene 8: 877–886.
Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E et al. (2003). Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med 9: 604–613.
Chen D, Xu LG, Chen L, Li L, Zhai Z, Shu HB . (2003). NIK is a component of the EGF/heregulin receptor signaling complexes. Oncogene 22: 4348–4355.
Feldkamp MM, Lau N, Rak J, Kerbel RS, Guha A . (1999). Normoxic and hypoxic regulation of vascular endothelial growth factor (VEGF) by astrocytoma cells is mediated by Ras. Int J Cancer 81: 118–124.
Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS et al. (1996). Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380: 439–442.
Garkavtsev I, Kozin SV, Chernova O, Xu L, Winkler F, Brown E et al. (2004). The candidate tumour suppressor protein ING4 regulates brain tumour growth and angiogenesis. Nature 428: 328–332.
Gille J, Swerlick RA, Caughman SW . (1997). Transforming growth factor-alpha-induced transcriptional activation of the vascular permeability factor (VPF/VEGF) gene requires AP-2-dependent DNA binding and transactivation. EMBO J 16: 750–759.
Heimberger AB, Hlatky R, Suki D, Yang D, Weinberg J, Gilbert M et al. (2005). Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res 11: 1462–1466.
Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P et al. (2007). Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445: 776–780.
Holland EC, Hively WP, DePinho RA, Varmus HE . (1998). A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev 12: 3675–3685.
Holtmann H, Winzen R, Holland P, Eickemeier S, Hoffmann E, Wallach D et al. (1999). Induction of interleukin-8 synthesis integrates effects on transcription and mRNA degradation from at least three different cytokine- or stress-activated signal transduction pathways. Mol Cell Biol 19: 6742–6753.
Huang HS, Nagane M, Klingbeil CK, Lin H, Nishikawa R, Ji XD et al. (1997). The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J Biol Chem 272: 2927–2935.
Hurtt MR, Moossy J, Donovan-Peluso M, Locker J . (1992). Amplification of epidermal growth factor receptor gene in gliomas: histopathology and prognosis. J Neuropathol Exp Neurol 51: 84–90.
Inda MM, Bonavia R, Mukasa A, Narita Y, Sah DW, Vandenberg S et al. (2010). Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes Dev 24: 1731–1745.
Jaros E, Perry RH, Adam L, Kelly PJ, Crawford PJ, Kalbag RM et al. (1992). Prognostic implications of p53 protein, epidermal growth factor receptor, and Ki-67 labelling in brain tumours. Br J Cancer 66: 373–385.
Jiang X, Takahashi N, Matsui N, Tetsuka T, Okamoto T . (2003). The NF-kappa B activation in lymphotoxin beta receptor signaling depends on the phosphorylation of p65 at serine 536. J Biological Chem 278: 919–926.
Jochum W, Passegue E, Wagner EF . (2001). AP-1 in mouse development and tumorigenesis. Oncogene 20: 2401–2412.
Karin M, Cao Y, Greten FR, Li ZW . (2002). NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2: 301–310.
Karin M, Lin A . (2002). NF-kappaB at the crossroads of life and death. Nat Immunol 3: 221–227.
Kyriakis JM, Avruch J . (2001). Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 81: 807–869.
Lal S, Lacroix M, Tofilon P, Fuller GN, Sawaya R, Lang FF . (2000). An implantable guide-screw system for brain tumor studies in small animals. J Neurosurg 92: 326–333.
Lee CH, Jeon YT, Kim SH, Song YS . (2007). NF-kappaB as a potential molecular target for cancer therapy. Biofactors 29: 19–35.
Le Page C, Koumakpayi IH, Lessard L, Saad F, Mes-Masson AM . (2005). Independent role of phosphoinositol-3-kinase (PI3K) and casein kinase II (CK-2) in EGFR and Her-2-mediated constitutive NF-kappaB activation in prostate cancer cells. Prostate 65: 306–315.
Li Q, Verma IM . (2002). NF-kappaB regulation in the immune system. Nat Rev Immunol 2: 725–734.
Magnus N, Garnier D, Rak J . (2010). Oncogenic epidermal growth factor receptor up-regulates multiple elements of the tissue factor signaling pathway in human glioma cells. Blood 116: 815–818.
Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK et al. (2001). Malignant glioma: genetics and biology of a grave matter. Genes Dev 15: 1311–1333.
Malliri A, Symons M, Hennigan RF, Hurlstone AF, Lamb RF, Wheeler T et al. (1998). The transcription factor AP-1 is required for EGF-induced activation of rho-like GTPases, cytoskeletal rearrangements, motility, and in vitro invasion of A431 cells. J Cell Biol 143: 1087–1099.
Matsusaka T, Fujikawa K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T et al. (1993). Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci USA 90: 10193–10197.
Nagane M, Levitzki A, Gazit A, Cavenee WK, Huang HJ . (1998). Drug resistance of human glioblastoma cells conferred by a tumor-specific mutant epidermal growth factor receptor through modulation of Bcl-XL and caspase-3-like proteases. Proc Natl Acad Sci USA 95: 5724–5729.
Narita Y, Nagane M, Mishima K, Huang HJ, Furnari FB, Cavenee WK . (2002). Mutant epidermal growth factor receptor signaling down-regulates p27 through activation of the phosphatidylinositol 3-kinase/Akt pathway in glioblastomas. Cancer Res 62: 6764–6769.
Nishikawa R, Ji XD, Harmon RC, Lazar CS, Gill GN, Cavenee WK et al. (1994). A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci USA 91: 7727–7731.
Nishikawa R, Sugiyama T, Narita Y, Furnari F, Cavenee WK, Matsutani M . (2004). Immunohistochemical analysis of the mutant epidermal growth factor, deltaEGFR, in glioblastoma. Brain Tumor Pathol 21: 53–56.
Norden AD, Drappatz J, Wen PY . (2008). Novel anti-angiogenic therapies for malignant gliomas. Lancet Neurol 7: 1152–1160.
Petit AM, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B et al. (1997). Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol 151: 1523–1530.
Prigent SA, Nagane M, Lin H, Huvar I, Boss GR, Feramisco JR et al. (1996). Enhanced tumorigenic behavior of glioblastoma cells expressing a truncated epidermal growth factor receptor is mediated through the Ras-Shc-Grb2 pathway. J Biol Chem 271: 25639–25645.
Romashkova JA, Makarov SS . (1999). NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature 401: 86–90.
Schlegel J, Merdes A, Stumm G, Albert FK, Forsting M, Hynes N et al. (1994). Amplification of the epidermal-growth-factor-receptor gene correlates with different growth behaviour in human glioblastoma. Int J Cancer 56: 72–77.
Shinojima N, Tada K, Shiraishi S, Kamiryo T, Kochi M, Nakamura H et al. (2003). Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res 63: 6962–6970.
Sparmann A, Bar-Sagi D . (2004). Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell 6: 447–458.
Takamori H, Oades ZG, Hoch OC, Burger M, Schraufstatter IU . (2000). Autocrine growth effect of IL-8 and GROalpha on a human pancreatic cancer cell line, Capan-1. Pancreas 21: 52–56.
Tsukahara T, Kannagi M, Ohashi T, Kato H, Arai M, Nunez G et al. (1999). Induction of Bcl-x(L) expression by human T-cell leukemia virus type 1 Tax through NF-kappaB in apoptosis-resistant T-cell transfectants with Tax. J Virol 73: 7981–7987.
Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM . (1996). Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science 274: 787–789.
van Dam H, Castellazzi M . (2001). Distinct roles of Jun: Fos and Jun: ATF dimers in oncogenesis. Oncogene 20: 2453–2464.
Wang ZQ, Grigoriadis AE, Mohle-Steinlein U, Wagner EF . (1991). A novel target cell for c-fos-induced oncogenesis: development of chondrogenic tumours in embryonic stem cell chimeras. EMBO J 10: 2437–2450.
Weissenberger J, Loeffler S, Kappeler A, Kopf M, Lukes A, Afanasieva TA et al. (2004). IL-6 is required for glioma development in a mouse model. Oncogene 23: 3308–3316.
Whitmarsh AJ, Davis RJ . (1996). Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med 74: 589–607.
Yao C, Lin Y, Chua MS, Ye CS, Bi J, Li W et al. (2007). Interleukin-8 modulates growth and invasiveness of estrogen receptor-negative breast cancer cells. Int J Cancer 121: 1949–1957.
Young MR, Li JJ, Rincon M, Flavell RA, Sathyanarayana BK, Hunziker R et al. (1999). Transgenic mice demonstrate AP-1 (activator protein-1) transactivation is required for tumor promotion. Proc Natl Acad Sci USA 96: 9827–9832.
Zhong Z, Wen Z, Darnell Jr JE . (1994). Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 264: 95–98.
Zhou Y, Larsen PH, Hao C, Yong VW . (2002). CXCR4 is a major chemokine receptor on glioma cells and mediates their survival. J Biol Chem 277: 49481–49487.
Acknowledgements
We thank Donna Harclerode and Donald Pizzo for technical assistance with immunostaining and Dr Cameron Brennan for providing the GBM tumorspheres. This work was supported by an award from the Goldhirsh Foundation (to FBF), and NIH Grant P01-CA95616 (to WKC, FBF), as well as NIH RO1CA130966, a grant with Pennsylvania Department of Health and an Innovative Research Scholar Award of the Hillman Foundation (to SYC). WKC is a fellow of the National Foundation for Cancer Research. MM Inda thanks the Gobierno de Navarra, Spain, and the American Brain Tumor Association in Honor of Walter Terlik for the fellowships received. R Bonavia was supported by a fellowship from Federazione Italiana Ricerca Cancro.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Bonavia, R., Inda, M., Vandenberg, S. et al. EGFRvIII promotes glioma angiogenesis and growth through the NF-κB, interleukin-8 pathway. Oncogene 31, 4054–4066 (2012). https://doi.org/10.1038/onc.2011.563
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.563
Keywords
This article is cited by
-
Natural products as promising targets in glioblastoma multiforme: a focus on NF-κB signaling pathway
Pharmacological Reports (2020)
-
Monocarboxylate transporter 1 and monocarboxylate transporter 4 in cancer-endothelial co-culturing microenvironments promote proliferation, migration, and invasion of renal cancer cells
Cancer Cell International (2019)
-
Upregulated expression of HOXB7 in intrahepatic cholangiocarcinoma is associated with tumor cell metastasis and poor prognosis
Laboratory Investigation (2019)
-
Epidermal growth factor receptor and EGFRvIII in glioblastoma: signaling pathways and targeted therapies
Oncogene (2018)
-
High expression of Bruton’s tyrosine kinase (BTK) is required for EGFR-induced NF-κB activation and predicts poor prognosis in human glioma
Journal of Experimental & Clinical Cancer Research (2017)