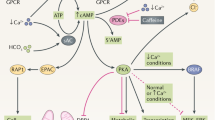
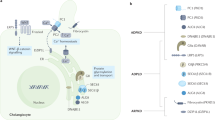
Key Points
-
For over a decade, the 'hallmarks of cancer' have provided a unifying framework for the complex set of cell, tissue and organismal defects associated with malignancy
-
Autosomal dominant polycystic kidney disease (ADPKD) is a relatively common inherited disorder with pathological features that echo those found in cancer
-
A systematic evaluation of ADPKD in the context of the ten cancer hallmarks emphasizes a surprising degree of similarity in signalling defects associated with the disease
-
Two key signalling processes involved in ADPKD—ciliary signalling and control of Ca2+/cAMP and polarized secretion—are not currently thought to be hallmarks of cancer
-
The pattern of conserved and distinct signalling pathways emphasizes important issues relevant to the optimal treatment of patients with ADPKD
Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is a progressive inherited disorder in which renal tissue is gradually replaced with fluid-filled cysts, giving rise to chronic kidney disease (CKD) and progressive loss of renal function. ADPKD is also associated with liver ductal cysts, hypertension, chronic pain and extra-renal problems such as cerebral aneurysms. Intriguingly, improved understanding of the signalling and pathological derangements characteristic of ADPKD has revealed marked similarities to those of solid tumours, even though the gross presentation of tumours and the greater morbidity and mortality associated with tumour invasion and metastasis would initially suggest entirely different disease processes. The commonalities between ADPKD and cancer are provocative, particularly in the context of recent preclinical and clinical studies of ADPKD that have shown promise with drugs that were originally developed for cancer. The potential therapeutic benefit of such repurposing has led us to review in detail the pathological features of ADPKD through the lens of the defined, classic hallmarks of cancer. In addition, we have evaluated features typical of ADPKD, and determined whether evidence supports the presence of such features in cancer cells. This analysis, which places pathological processes in the context of defined signalling pathways and approved signalling inhibitors, highlights potential avenues for further research and therapeutic exploitation in both diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Torres, V. E. & Harris, P. C. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 76, 149–168 (2009).
Weir, B., Zhao, X. & Meyerson, M. Somatic alterations in the human cancer genome. Cancer Cell 6, 433–438 (2004).
Howlader, N. et al. SEER cancer statistics review, 1975–2011. National Cancer Institute [online], (2014).
Grantham, J. J. Polycystic kidney disease: neoplasia in disguise. Am. J. Kidney Dis. 15, 110–116 (1990).
Paul, S. M. et al. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat. Rev. Drug Discov. 9, 203–214 (2010).
Mestre-Ferrandiz, J., Sussex, J. & Towse, A. The R&D cost of a new medicine. (Office of Health Economics, 2012).
Grabowski, H. G. & Hansen, R. W. Innovation in the pharmaceutical industry: new estimates of R&D costs [online], (2014).
Seeger-Nukpezah, T. et al. Inhibiting the HSP90 chaperone slows cyst growth in a mouse model of autosomal dominant polycystic kidney disease. Proc. Natl Acad. Sci. USA 110, 12786–12791 (2013).
Bukanov, N. O. et al. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature 444, 949–952 (2006).
Sweeney, W. E. Jr, et al. Src inhibition ameliorates polycystic kidney disease. J. Am. Soc. Nephrol. 19, 1331–1341 (2008).
Serra, A. L. et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N. Engl. J. Med. 363, 820–829 (2010).
Walz, G. et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 363, 830–840 (2010).
Cao, Y. et al. Chemical modifier screen identifies HDAC inhibitors as suppressors of PKD models. Proc. Natl Acad. Sci. USA 106, 21819–21824 (2009).
Leuenroth, S. J. et al. Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. Proc. Natl Acad. Sci. USA 104, 4389–4394 (2007).
Omori, S. et al. Extracellular signal-regulated kinase inhibition slows disease progression in mice with polycystic kidney disease. J. Am. Soc. Nephrol. 17, 1604–1614 (2006).
Gallagher, A. R., Germino, G. G. & Somlo, S. Molecular advances in autosomal dominant polycystic kidney disease. Adv. Chronic Kidney Dis. 17, 118–130 (2010).
Chapin, H. C. & Caplan, M. J. The cell biology of polycystic kidney disease. J. Cell Biol. 191, 701–710 (2010).
Harris, P. C. & Torres, V. E. Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J. Clin. Invest. 124, 2315–2324 (2014).
Grantham, J. J., Mulamalla, S. & Swenson-Fields, K. I. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat. Rev. Nephrol. 7, 556–566 (2011).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Grantham, J. J., Geiser, J. L. & Evan, A. P. Cyst formation and growth in autosomal dominant polycystic kidney disease. Kidney Int. 31, 1145–1152 (1987).
Nadasdy, T. et al. Proliferative activity of cyst epithelium in human renal cystic diseases. J. Am. Soc. Nephrol. 5, 1462–1468 (1995).
Lantinga-van Leeuwen, I. S. et al. Kidney-specific inactivation of the Pkd1 gene induces rapid cyst formation in developing kidneys and a slow onset of disease in adult mice. Hum. Mol. Genet. 16, 3188–3196 (2007).
Wilson, S. J. et al. Inhibition of HER-2(neu/ErbB2) restores normal function and structure to polycystic kidney disease (PKD) epithelia. Biochim. Biophys. Acta 1762, 647–655 (2006).
Nakanishi, K. et al. Renal dysfunction but not cystic change is ameliorated by neonatal epidermal growth factor in bpk mice. Pediatr. Nephrol. 16, 45–50 (2001).
Yamaguchi, T. et al. Sorafenib inhibits cAMP-dependent ERK activation, cell proliferation, and in vitro cyst growth of human ADPKD cyst epithelial cells. Am. J. Physiol. Renal Physiol. 299, F944–F951 (2010).
Yamaguchi, T. et al. cAMP stimulates the in vitro proliferation of renal cyst epithelial cells by activating the extracellular signal-regulated kinase pathway. Kidney Int. 57, 1460–1471 (2000).
Shillingford, J. M. et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc. Natl Acad. Sci. USA 103, 5466–5471 (2006).
Shillingford, J. M. et al. Rapamycin ameliorates PKD resulting from conditional inactivation of Pkd1. J. Am. Soc. Nephrol. 21, 489–497 (2010).
Weimbs, T., Olsan, E. E. & Talbot, J. J. Regulation of STATs by polycystin-1 and their role in polycystic kidney disease. JAKSTAT 2, e23650 (2013).
Cowley, B. D. Jr, et al. Elevated proto-oncogene expression in polycystic kidneys of the C57BL/6J (cpk) mouse. J. Am. Soc. Nephrol. 1, 1048–1053 (1991).
Nakamura, T. et al. Growth factor gene expression in kidney of murine polycystic kidney disease. J. Am. Soc. Nephrol. 3, 1378–1386 (1993).
Trudel, M., D'Agati, V. & Costantini, F. C-myc as an inducer of polycystic kidney disease in transgenic mice. Kidney Int. 39, 665–671 (1991).
Piontek, K. et al. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat. Med. 13, 1490–1495 (2007).
Takakura, A. et al. Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum. Mol. Genet. 18, 2523–2531 (2009).
Patel, V. et al. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum. Mol. Genet. 17, 1578–1590 (2008).
Shibazaki, S. et al. Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum. Mol. Genet. 17, 1505–1516 (2008).
Levental, K. R. et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 (2009).
Butcher, D. T., Alliston, T. & Weaver, V. M. A tense situation: forcing tumour progression. Nat. Rev. Cancer 9, 108–122 (2009).
Norman, J. Fibrosis and progression of autosomal dominant polycystic kidney disease (ADPKD). Biochim. Biophys. Acta 1812, 1327–1336 (2011).
Nuzzo, P. V. et al. Periostin: a novel prognostic and therapeutic target for genitourinary cancer? Clin. Genitourin. Cancer 12, 301–311 (2014).
Wallace, D. P. et al. Periostin promotes renal cyst growth and interstitial fibrosis in polycystic kidney disease. Kidney Int. 85, 845–854 (2014).
Lee, K. et al. Inactivation of integrin-β1 prevents the development of polycystic kidney disease after the loss of polycystin-1. J. Am. Soc. Nephrol. http://dx.doi.org/10.1681/ASN.2013111179.
Seeger-Nukpezah, T. & Golemis, E. A. The extracellular matrix and ciliary signaling. Curr. Opin. Cell Biol. 24, 652–661 (2012).
Nishio, S. et al. Pkd1 regulates immortalized proliferation of renal tubular epithelial cells through p53 induction and JNK activation. J. Clin. Invest. 115, 910–918 (2005).
Zhou, X. et al. Sirtuin 1 inhibition delays cyst formation in autosomal-dominant polycystic kidney disease. J. Clin. Invest. 123, 3084–3098 (2013).
Fan, L. X. et al. Inhibition of histone deacetylases targets the transcription regulator Id2 to attenuate cystic epithelial cell proliferation. Kidney Int. 81, 76–85 (2012).
Bhunia, A. K. et al. PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell 109, 157–168 (2002).
Park, J. Y. et al. p21 is decreased in polycystic kidney disease and leads to increased epithelial cell cycle progression: roscovitine augments p21 levels. BMC Nephrol. 8, 12 (2007).
Felekkis, K. N. et al. Mutant polycystin-2 induces proliferation in primary rat tubular epithelial cells in a STAT-1/p21-independent fashion accompanied instead by alterations in expression of p57KIP2 and Cdk2. BMC Nephrol. 9, 10 (2008).
The European Polycystic Kidney Disease Consortium. The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell 77, 881–894 (1994).
Brook-Carter, P. T. et al. Deletion of the TSC2 and PKD1 genes associated with severe infantile polycystic kidney disease—a contiguous gene syndrome. Nat. Genet. 8, 328–332 (1994).
Boletta, A. Emerging evidence of a link between the polycystins and the mTOR pathways. Pathogenetics 2, 6 (2009).
Boehlke, C. et al. Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nat. Cell Biol. 12, 1115–1122 (2010).
Rowe, I. et al. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat. Med. 19, 488–493 (2013).
Varelas, X. et al. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev. Cell 18, 579–591 (2010).
Habbig, S. et al. NPHP4, a cilia-associated protein, negatively regulates the Hippo pathway. J. Cell Biol. 193, 633–642 (2011).
Pan, D. The hippo signaling pathway in development and cancer. Dev. Cell 19, 491–505 (2010).
Ganem, N. J. et al. Cytokinesis failure triggers hippo tumor suppressor pathway activation. Cell 158, 833–848 (2014).
Adams, J. M. & Cory, S. The Bcl-2 protein family: arbiters of cell survival. Science 281, 1322–1326 (1998).
Sayers, T. J. Targeting the extrinsic apoptosis signaling pathway for cancer therapy. Cancer Immunol. Immunother. 60, 1173–1180 (2011).
Marino, G. et al. Self-consumption: the interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 15, 81–94 (2014).
Galluzzi, L. et al. Molecular mechanisms of regulated necrosis. Semin. Cell Dev. Biol. 35, 24–32 (2014).
Vanden Berghe, T. et al. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 15, 135–147 (2014).
Fan, L. X. et al. Smac-mimetic-induced epithelial cell death reduces the growth of renal cysts. J. Am. Soc. Nephrol. 24, 2010–2022 (2013).
Goilav, B. Apoptosis in polycystic kidney disease. Biochim. Biophys. Acta 1812, 1272–1280 (2011).
Woo, D. Apoptosis and loss of renal tissue in polycystic kidney diseases. N. Engl. J. Med. 333, 18–25 (1995).
Veis, D. J. et al. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 75, 229–240 (1993).
Hughes, P. et al. Loss of PKD1 and loss of Bcl-2 elicit polycystic kidney disease through distinct mechanisms. Cell Death Differ. 13, 1123–1127 (2006).
Yu, W. et al. Polycystin-1 protein level determines activity of the Galpha12/JNK apoptosis pathway. J. Biol. Chem. 285, 10243–10251 (2010).
Tao, Y. et al. Caspase inhibition reduces tubular apoptosis and proliferation and slows disease progression in polycystic kidney disease. Proc. Natl Acad. Sci. USA 102, 6954–6959 (2005).
Pampliega, O. et al. Functional interaction between autophagy and ciliogenesis. Nature 502, 194–200 (2013).
Tang, Z. et al. Autophagy promotes primary ciliogenesis by removing OFD1 from centriolar satellites. Nature 502, 254–257 (2013).
Ravichandran, K. & Edelstein, C. L. Polycystic kidney disease: a case of suppressed autophagy? Semin. Nephrol. 34, 27–33 (2014).
Li, X. et al. A tumor necrosis factor-α-mediated pathway promoting autosomal dominant polycystic kidney disease. Nat. Med. 14, 863–868 (2008).
Coschi, C. H. & Dick, F. A. Chromosome instability and deregulated proliferation: an unavoidable duo. Cell. Mol. Life Sci. 69, 2009–2024 (2012).
Lopez-Otin, C. et al. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Verdin, E. The many faces of sirtuins: coupling of NAD metabolism, sirtuins and lifespan. Nat. Med. 20, 25–27 (2014).
Roth, M. & Chen, W. Y. Sorting out functions of sirtuins in cancer. Oncogene 33, 1609–1620 (2014).
Parker, E. et al. Hyperproliferation of PKD1 cystic cells is induced by insulin-like growth factor-1 activation of the Ras/Raf signalling system. Kidney Int. 72, 157–165 (2007).
Schaffner, D. L. et al. Targeting of the rasT24 oncogene to the proximal convoluted tubules in transgenic mice results in hyperplasia and polycystic kidneys. Am. J. Pathol. 142, 1051–1060 (1993).
Yamaguchi, T. et al. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J. Biol. Chem. 279, 40419–40430 (2004).
Kim, W. Y. & Kaelin, W. G. Role of VHL gene mutation in human cancer. J. Clin. Oncol. 22, 4991–5004 (2004).
Rankin, E. B., Tomaszewski, J. E. & Haase, V. H. Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res. 66, 2576–2583 (2006).
Wang, S. S. et al. Bap1 is essential for kidney function and cooperates with Vhl in renal tumorigenesis. Proc. Natl Acad. Sci. USA 111, 16538–16543 (2014).
Young, A. P. et al. VHL loss actuates a HIF-independent senescence programme mediated by Rb and p400. Nat. Cell Biol. 10, 361–369 (2008).
Muller, R. U. et al. The von Hippel Lindau tumor suppressor limits longevity. J. Am. Soc. Nephrol. 20, 2513–2517 (2009).
Mehta, R. et al. Proteasomal regulation of the hypoxic response modulates aging in C. elegans. Science 324, 1196–1198 (2009).
Pirson, Y. Extrarenal manifestations of autosomal dominant polycystic kidney disease. Adv. Chronic Kidney Dis. 17, 173–180 (2010).
Bello-Reuss, E., Holubec, E. K. & Rajaraman, S. Angiogenesis in autosomal-dominant polycystic kidney disease. Kidney Int. 60, 37–45 (2001).
Wei, W. et al. Evidence of angiogenesis and microvascular regression in autosomal-dominant polycystic kidney disease kidneys: a corrosion cast study. Kidney Int. 70, 1261–1268 (2006).
Bernhardt, W. M. et al. Involvement of hypoxia-inducible transcription factors in polycystic kidney disease. Am. J. Pathol. 170, 830–842 (2007).
Tao, Y. et al. VEGF receptor inhibition slows the progression of polycystic kidney disease. Kidney Int. 72, 1358–1366 (2007).
Belibi, F. et al. Hypoxia-inducible factor-1α (HIF-1α) and autophagy in polycystic kidney disease (PKD). Am. J. Physiol. Renal Physiol. 300, F1235–F1243 (2011).
Reed, B. Y. et al. Angiogenic growth factors correlate with disease severity in young patients with autosomal dominant polycystic kidney disease. Kidney Int. 79, 128–134 (2011).
Kim, K. et al. Polycystin 1 is required for the structural integrity of blood vessels. Proc. Natl Acad. Sci. USA 97, 1731–1736 (2000).
Outeda, P. et al. Polycystin signaling is required for directed endothelial cell migration and lymphatic development. Cell Rep. 7, 634–644 (2014).
Rowe, I. et al. Impaired glomerulogenesis and endothelial cell migration in Pkd1-deficient renal organ cultures. Biochem. Biophys. Res. Commun. 444, 473–479 (2014).
Coxam, B. et al. Pkd1 regulates lymphatic vascular morphogenesis during development. Cell Rep. 7, 623–633 (2014).
Raina, S. et al. Anti-VEGF antibody treatment accelerates polycystic kidney disease. Am. J. Physiol. Renal Physiol. 301, F773–F783 (2011).
Bensinger, S. J. & Christofk, H. R. New aspects of the Warburg effect in cancer cell biology. Semin. Cell Dev. Biol. 23, 352–361 (2012).
Rowe, I. & Boletta, A. Defective metabolism in polycystic kidney disease: potential for therapy and open questions. Nephrol. Dial. Transplant. 29, 1480–1486 (2014).
Mao, Z., Xie, G. & Ong, A. C. Metabolic abnormalities in autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 30, 197–203 (2014).
Wang, X. et al. Targeting of sodium-glucose cotransporters with phlorizin inhibits polycystic kidney disease progression in Han:SPRD rats. Kidney Int. 84, 962–968 (2013).
Eagle, H. Nutrition needs of mammalian cells in tissue culture. Science 122, 501–514 (1955).
DeBerardinis, R. J. et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl Acad. Sci. USA 104, 19345–19350 (2007).
Lukey, M. J., Wilson, K. F. & Cerione, R. A. Therapeutic strategies impacting cancer cell glutamine metabolism. Future Med. Chem. 5, 1685–1700 (2013).
Lieberman, B. P. et al. PET imaging of glutaminolysis in tumors by 18F-(2S, 4R)4-fluoroglutamine. J. Nucl. Med. 52, 1947–1955 (2011).
Hanahan, D. & Coussens, L. M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 (2012).
Werder, A. A. et al. Comparative effects of germfree and ambient environments on the development of cystic kidney disease in CFWwd mice. J. Lab. Clin. Med. 103, 399–407 (1984).
Gardner, K. D. Jr et al. Cytokines in fluids from polycystic kidneys. Kidney Int. 39, 718–724 (1991).
Merta, M. et al. Cytokine profile in autosomal dominant polycystic kidney disease. Biochem. Mol. Biol. Int. 41, 619–624 (1997).
Cowley, B. D. Jr et al. Increased renal expression of monocyte chemoattractant protein-1 and osteopontin in ADPKD in rats. Kidney Int. 60, 2087–2096 (2001).
Karihaloo, A. et al. Macrophages promote cyst growth in polycystic kidney disease. J. Am. Soc. Nephrol. 22, 1809–1814 (2011).
Swenson-Fields, K. I. et al. Macrophages promote polycystic kidney disease progression. Kidney Int. 83, 855–864 (2013).
Mrug, M. et al. Overexpression of innate immune response genes in a model of recessive polycystic kidney disease. Kidney Int. 73, 63–76 (2008).
Zhou, J. et al. Kidney injury accelerates cystogenesis via pathways modulated by heme oxygenase and complement. J. Am. Soc. Nephrol. 23, 1161–1171 (2012).
Brasier, J. L. & Henske, E. P. Loss of the polycystic kidney disease (PKD1) region of chromosome 16p13 in renal cyst cells supports a loss-of-function model for cyst pathogenesis. J. Clin. Invest. 99, 194–199 (1997).
Torra, R. et al. A loss-of-function model for cystogenesis in human autosomal dominant polycystic kidney disease type 2. Am. J. Hum. Genet. 65, 345–352 (1999).
Pei, Y. et al. Somatic PKD2 mutations in individual kidney and liver cysts support a “two-hit” model of cystogenesis in type 2 autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 10, 1524–1529 (1999).
Knudson, A. G. Jr, Hethcote, H. W. & Brown, B. W. Mutation and childhood cancer: a probabilistic model for the incidence of retinoblastoma. Proc. Natl Acad. Sci. USA 72, 5116–5120 (1975).
Woo, Y. M. et al. Genome-wide methylation profiling of ADPKD identified epigenetically regulated genes associated with renal cyst development. Hum. Genet. 133, 281–297 (2014).
Battini, L. et al. Loss of polycystin-1 causes centrosome amplification and genomic instability. Hum. Mol. Genet. 17, 2819–2833 (2008).
Aboualaiwi, W. A. et al. Survivin-induced abnormal ploidy contributes to cystic kidney and aneurysm formation. Circulation 129, 660–672 (2014).
AbouAlaiwi, W. A. et al. Endothelial cells from humans and mice with polycystic kidney disease are characterized by polyploidy and chromosome segregation defects through survivin down-regulation. Hum. Mol. Genet. 20, 354–367 (2011).
Li, M. et al. Genomic instability in patients with autosomal-dominant polycystic kidney disease. J. Int. Med. Res. 41, 169–175 (2013).
Reynolds, D. M. et al. Aberrant splicing in the PKD2 gene as a cause of polycystic kidney disease. J. Am. Soc. Nephrol. 10, 2342–2351 (1999).
Aguiari, G. et al., Deficiency of polycystin-2 reduces Ca2+ channel activity and cell proliferation in ADPKD lymphoblastoid cells. FASEB J. 18, 884–886 (2004).
Rossetti, S. et al. Autosomal dominant polycystic kidney disease (ADPKD) in an Italian family carrying a novel nonsense mutation and two missense changes in exons 44 and 45 of the PKD1 gene. Am. J. Med. Genet. 65, 155–159 (1996).
Chaki, M. et al. Exome capture reveals ZNF423 and CEP164 mutations, linking renal ciliopathies to DNA damage response signaling. Cell 150, 533–548 (2012).
Choi, H. J. et al. NEK8 links the ATR-regulated replication stress response and S phase CDK activity to renal ciliopathies. Mol. Cell 51, 423–439 (2013).
Airik, R. et al. Renal-retinal ciliopathy gene Sdccag8 Regulates DNA damage response signaling. J. Am. Soc. Nephrol. 25, 2573–2583 (2014).
Kaucka, M. et al. The planar cell polarity pathway drives pathogenesis of chronic lymphocytic leukemia by the regulation of B-lymphocyte migration. Cancer Res. 73, 1491–1501 (2013).
Luga, V. et al. Exosomes mediate stromal mobilization of autocrine Wnt–PCP signaling in breast cancer cell migration. Cell 151, 1542–1556 (2012).
Katoh, M. WNT/PCP signaling pathway and human cancer. Oncol. Rep, 14, 1583–1588 (2005).
Cui, C. et al. Wdpcp, a PCP protein required for ciliogenesis, regulates directional cell migration and cell polarity by direct modulation of the actin cytoskeleton. PLoS Biol. 11, e1001720 (2013).
Lienkamp, S., Ganner, A. & Walz, G. Inversin, Wnt signaling and primary cilia. Differentiation 83, S49–S55 (2012).
Wilson, P. D. Apico-basal polarity in polycystic kidney disease epithelia. Biochim. Biophys. Acta 1812, 1239–1248 (2011).
Goggolidou, P. Wnt and planar cell polarity signaling in cystic renal disease. Organogenesis 10, 86–95 (2014).
Fedeles, S. & Gallagher, A. R. Cell polarity and cystic kidney disease. Pediatr. Nephrol. 28, 1161–1172 (2013).
Lancaster, M. A. et al. Impaired Wnt-β-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nat. Med. 15, 1046–1054 (2009).
Castelli, M. et al. Polycystin-1 binds Par3/aPKC and controls convergent extension during renal tubular morphogenesis. Nat. Commun. 4, 2658 (2013).
Boca, M. et al. Polycystin-1 induces cell migration by regulating phosphatidylinositol 3-kinase-dependent cytoskeletal rearrangements and GSK3β-dependent cell–cell mechanical adhesion. Mol. Biol. Cell 18, 4050–4061 (2007).
Luyten, A. et al. Aberrant regulation of planar cell polarity in polycystic kidney disease. J. Am. Soc. Nephrol. 21, 1521–1532 (2010).
Pease, J. C. & Tirnauer, J. S. Mitotic spindle misorientation in cancer—out of alignment and into the fire. J. Cell Sci. 124, 1007–1016 (2011).
Delaval, B. et al. The cilia protein IFT88 is required for spindle orientation in mitosis. Nat. Cell Biol. 13, 461–468 (2011).
Sugiyama, N. et al. The canonical Wnt signaling pathway is not involved in renal cyst development in the kidneys of inv mutant mice. Kidney Int. 79, 957–965 (2011).
Jonassen, J. A. et al. Disruption of IFT complex A causes cystic kidneys without mitotic spindle misorientation. J. Am. Soc. Nephrol. 23, 641–651 (2012).
Nishio, S. et al. Loss of oriented cell division does not initiate cyst formation. J. Am. Soc. Nephrol. 21, 295–302 (2010).
Massague, J. TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 13, 616–630 (2012).
Markoff, A. et al. Annexin A5 interacts with polycystin-1 and interferes with the polycystin-1 stimulated recruitment of E-cadherin into adherens junctions. J. Mol. Biol. 369, 954–966 (2007).
Charron, A. J. et al. Compromised cytoarchitecture and polarized trafficking in autosomal dominant polycystic kidney disease cells. J. Cell Biol. 149, 111–124 (2000).
Chea, S. W. & Lee, K. B. TGF-β mediated epithelial-mesenchymal transition in autosomal dominant polycystic kidney disease. Yonsei Med. J. 50, 105–111 (2009).
Togawa, H. et al. Epithelial-to-mesenchymal transition in cyst lining epithelial cells in an orthologous PCK rat model of autosomal-recessive polycystic kidney disease. Am. J. Physiol. Renal Physiol. 300, F511–F520 (2011).
You, N. et al. Tg737 signaling is required for hypoxia-enhanced invasion and migration of hepatoma cells. J. Exp. Clin. Cancer Res. 31, 75 (2012).
Nikonova, A. S. et al. Nedd9 restrains renal cystogenesis in Pkd1−/− mice. Proc. Natl Acad. Sci. USA 111, 12859–12864 (2014).
Elliott, J., Zheleznova, N. N. & Wilson, P. D. c-Src inactivation reduces renal epithelial cell-matrix adhesion, proliferation, and cyst formation. Am. J. Physiol. Cell Physiol. 301, C522–529 (2011).
Barr, M. M. et al. The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr. Biol. 11, 1341–1346 (2001).
Yoder, B. K., Hou, X. & Guay-Woodford, L. M. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J. Am. Soc. Nephrol. 13, 2508–2516 (2002).
Hildebrandt, F., Benzing, T. & Katsanis, N. Ciliopathies. N. Engl. J. Med. 364, 1533–1543 (2011).
Yoder, B. K. et al. Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am. J. Physiol. Renal Physiol. 282, F541–F552 (2002).
Sun, Z. et al. A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development 131, 4085–4093 (2004).
Frew, I. J. et al. pVHL and PTEN tumour suppressor proteins cooperatively suppress kidney cyst formation. EMBO J. 27, 1747–1757 (2008).
Ma, M. et al. Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat. Genet. 45, 1004–1012 (2013).
Hassounah, N. B., Bunch, T. A. & McDermott, K. M. Molecular pathways: the role of primary cilia in cancer progression and therapeutics with a focus on Hedgehog signaling. Clin. Cancer Res. 18, 2429–2435 (2012).
Seeger-Nukpezah, T., Little, J. L., Serzhanova, V. & Golemis, E. A. Cilia and cilia-associated proteins in cancer. Drug Discov. Today Dis. Mech. 10, e135–e142 (2013).
Schraml, P. et al. Sporadic clear cell renal cell carcinoma but not the papillary type is characterized by severely reduced frequency of primary cilia. Mod. Pathol. 22, 31–36 (2009).
Emoto, K. et al. Presence of primary cilia in cancer cells correlates with prognosis of pancreatic ductal adenocarcinoma. Hum. Pathol. 45, 817–825 (2014).
Hassounah, N. B. et al. Primary cilia are lost in preinvasive and invasive prostate cancer. PLoS ONE 8, e68521 (2013).
Menzl, I. et al. Loss of primary cilia occurs early in breast cancer development. Cilia 3, 7 (2014).
Plotnikova, O. V., Golemis, E. A. & Pugacheva, E. N. Cell cycle-dependent ciliogenesis and cancer. Cancer Res. 68, 2058–2061 (2008).
Gradilone, S. A. et al. HDAC6 inhibition restores ciliary expression and decreases tumor growth. Cancer Res. 73, 2259–2270 (2013).
Han, Y. G. et al. Dual and opposing roles of primary cilia in medulloblastoma development. Nat. Med. 15, 1062–1065 (2009).
Kim, S. & Tsiokas, L. Cilia and cell cycle re-entry: more than a coincidence. Cell Cycle 10, 2683–2690 (2011).
Pan, J., Seeger-Nukpezah, T. & Golemis, E. A. The role of the cilium in normal and abnormal cell cycles: emphasis on renal cystic pathologies. Cell. Mol. Life Sci. 70, 1849–1874 (2013).
Nikonova, A. S. et al. Aurora A kinase (AURKA) in normal and pathological cell division. Cell. Mol. Life Sci. 70, 661–687 (2013).
Pugacheva, E. N. et al. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 129, 1351–1363 (2007).
Kaelin, W. G. Jr. . The von Hippel-Lindau tumor suppressor gene and kidney cancer. Clin. Cancer Res. 10 (18 Pt 2), 6290S–6295S (2004).
Maxwell, P. H. et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 (1999).
Na, X. et al. Identification of the RNA polymerase II subunit hsRPB7 as a novel target of the von Hippel-Lindau protein. EMBO J. 22, 4249–4259 (2003).
Shen, C. & Kaelin, W. G. Jr. The VHL/HIF axis in clear cell renal carcinoma. Semin. Cancer Biol. 23, 18–25 (2013).
Kim, S. H. et al. Human enhancer of filamentation 1 Is a mediator of hypoxia-inducible factor-1α-mediated migration in colorectal carcinoma cells. Cancer Res. 70, 4054–4063 (2010).
Xu, J. et al. VHL inactivation induces HEF1 and Aurora kinase A. J. Am. Soc. Nephrol. 21, 2041–2046 (2010).
Kuehn, E. W., Walz, G. & Benzing, T. Von hippel-lindau: a tumor suppressor links microtubules to ciliogenesis and cancer development. Cancer Res. 67, 4537–4540 (2007).
Huseman, R. et al. Macropuncture study of polycystic disease in adult human kidneys. Kidney Int. 18, 375–385 (1980).
Terryn, S. et al. Fluid transport and cystogenesis in autosomal dominant polycystic kidney disease. Biochim. Biophys. Acta 1812, 1314–1321 (2011).
Alper, S. L. Let's look at cysts from both sides now. Kidney Int. 74, 699–702 (2008).
Yamaguchi, T. et al. Renal accumulation and excretion of cyclic adenosine monophosphate in a murine model of slowly progressive polycystic kidney disease. Am. J. Kidney Dis. 30, 703–709 (1997).
Wallace, D. P. Cyclic AMP-mediated cyst expansion. Biochim. Biophys. Acta 1812, 1291–1300 (2011).
Yamaguchi, T. et al. Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J. Am. Soc. Nephrol. 17, 178–187 (2006).
Roderick, H. L. & Cook, S. J. Ca2+ signalling checkpoints in cancer: remodelling Ca2+ for cancer cell proliferation and survival. Nat. Rev. Cancer 8, 361–375 (2008).
Gold, M. G., Gonen, T. & Scott, J. D. Local cAMP signaling in disease at a glance. J. Cell Sci. 126, 4537–4543 (2013).
Torres, V. E. et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 367, 2407–2418 (2012).
Chang, M. Y. & Ong, A. C. Mechanism-based therapeutics for autosomal dominant polycystic kidney disease: recent progress and future prospects. Nephron Clin. Pract. 120, c25–c34 (2012).
FDA Drug Safety Communication: FDA limits duration and usage of Samsca (tolvaptan) due to possible liver injury leading to organ transplant or death. US Department of Health and Human Services [online], (2013).
Sontheimer, H. An unexpected role for ion channels in brain tumor metastasis. Exp. Biol. Med. (Maywood) 233, 779–791 (2008).
Haas, B. R. & Sontheimer, H. Inhibition of the sodium-potassium-chloride cotransporter isoform-1 reduces glioma invasion. Cancer Res. 70, 5597–5606 (2010).
Garzon-Muvdi, T. et al. Regulation of brain tumor dispersal by NKCC1 through a novel role in focal adhesion regulation. PLoS Biol. 10, e1001320 (2012).
Johnson, M. D. & O'Connell, M. Na-K-2Cl cotransporter and aquaporin 1 in arachnoid granulations, meningiomas, and meningiomas invading dura. Hum. Pathol. 44, 1118–1124 (2013).
D'Alessandro, G. et al. KCa3.1 channels are involved in the infiltrative behavior of glioblastoma in vivo. Cell Death Dis. 4, e773 (2013).
Verkman, A. S., Hara-Chikuma, M. & Papadopoulos, M. C. Aquaporins—new players in cancer biology. J. Mol. Med. (Berl.) 86, 523–529 (2008).
Xie, C. et al. CFTR suppresses tumor progression through miR-193b targeting urokinase plasminogen activator (uPA) in prostate cancer. Oncogene 32, 2282–2291 (2013).
North, W. G. et al. Expression of all known vasopressin receptor subtypes by small cell tumors implies a multifaceted role for this neuropeptide. Cancer Res. 58, 1866–1871 (1998).
Hemal, A. K. et al. Renal cell carcinoma in cases of adult polycystic kidney disease: changing diagnostic and therapeutic implications. Urol. Int. 64, 9–12 (2000).
Keith, D. S. et al. Renal cell carcinoma in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 4, 1661–1669 (1994).
Chen, Y. B. & Tickoo, S. K. Spectrum of preneoplastic and neoplastic cystic lesions of the kidney. Arch. Pathol. Lab. Med. 136, 400–409 (2012).
Orskov, B. et al. Changes in causes of death and risk of cancer in Danish patients with autosomal dominant polycystic kidney disease and end-stage renal disease. Nephrol. Dial. Transplant. 27, 1607–1613 (2012).
Grantham, J. J. Clinical practice. Autosomal dominant polycystic kidney disease. N. Engl. J. Med. 359, 1477–1485 (2008).
Bonsib, S. M. Renal cystic diseases and renal neoplasms: a mini-review. Clin. J. Am. Soc. Nephrol. 4, 1998–2007 (2009).
Hajj, P. et al. Prevalence of renal cell carcinoma in patients with autosomal dominant polycystic kidney disease and chronic renal failure. Urology 74, 631–634 (2009).
Wetmore, J. B. et al. Polycystic kidney disease and cancer after renal transplantation. J. Am. Soc. Nephrol. 25, 2335–2341 (2014).
Ward, C. J. et al. Germline PKHD1 mutations are protective against colorectal cancer. Hum. Genet. 129, 345–349 (2011).
Gargalionis, A. N. et al. Polycystin-1 and polycystin-2 are involved in the acquisition of aggressive phenotypes in colorectal cancer. Int. J. Cancer 136, 1515–1527 (2014).
Shuch, B. et al. Understanding pathologic variants of renal cell carcinoma: distilling therapeutic opportunities from biologic complexity. Eur. Urol. 67, 85–97 (2014).
Srigley, J. R. et al. The International Society of Urological Pathology (ISUP) Vancouver Classification of renal neoplasia. Am. J. Surg. Pathol. 37, 1469–1489 (2013).
Martinez, J. R. & Grantham, J. J. Polycystic kidney disease: etiology, pathogenesis, and treatment. Dis. Mon. 41, 693–765 (1995).
Sircar, K. & Tamboli, P. in Kidney Cancer: Principles and Practice (eds. Lara, P. N. Jr & Jonasch, E.) 17–28 (Springer–Verlag, 2012).
Neumann, H. P. & Zbar, B. Renal cysts, renal cancer and von Hippel-Lindau disease. Kidney Int. 51, 16–26 (1997).
Warren, K. S. & McFarlane, J. The Bosniak classification of renal cystic masses. BJU Int. 95, 939–942 (2005).
Zhang, J. et al. Diagnosis and treatment of cystic renal cell carcinoma. World J. Surg. Oncol. 11, 158 (2013).
Ellimoottil, C. et al. New modalities for the evaluation and surveillance of complex renal cysts. J. Urol. 192, 1604–1611 (2014).
The National Cancer Institute and National Human Genome Research Institute. The Cancer Genome Atlas [online], (2015).
Bastos, A. P. et al. Pkd1 haploinsufficiency increases renal damage and induces microcyst formation following ischemia/reperfusion. J. Am. Soc. Nephrol. 20, 2389–2402 (2009).
Durinck, S. et al. Spectrum of diverse genomic alterations define non-clear cell renal carcinoma subtypes. Nat. Genet. 47, 13–21. (2014).
Latif, F. et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science 260, 1317–1320 (1993).
Young, A. C. et al. Analysis of VHL Gene Alterations and their Relationship to Clinical Parameters in Sporadic Conventional Renal Cell Carcinoma. Clin. Cancer Res. 15, 7582–7592 (2009).
Nickerson, M. L. et al. Improved identification of von Hippel–Lindau gene alterations in clear cell renal tumors. Clin. Cancer Res. 14, 4726–4734 (2008).
Surveillance, Epidemiology, and End Results Program. SEER stat fact sheets: kidney and renal pelvis cancer. National Cancer Institute [online], (2014).
Solomon, D. & Schwartz, A. Renal pathology in von Hippel-Lindau disease. Hum. Pathol. 19, 1072–1079 (1988).
Lubensky, I. A. et al. Allelic deletions of the VHL gene detected in multiple microscopic clear cell renal lesions in von Hippel–Lindau disease patients. Am. J. Pathol. 149, 2089–2094 (1996).
Mandriota, S. J. et al. HIF activation identifies early lesions in VHL kidneys: evidence for site-specific tumor suppressor function in the nephron. Cancer Cell 1, 459–468 (2002).
Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 499, 43–49 (2013).
Montani, M. et al. VHL-gene deletion in single renal tubular epithelial cells and renal tubular cysts: further evidence for a cyst-dependent progression pathway of clear cell renal carcinoma in von Hippel–Lindau disease. Am. J. Surg. Pathol. 34, 806–815 (2010).
Sato, Y. et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat. Genet. 45, 860–867 (2013).
Varela, I. et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature 469, 539–542 (2011).
Pena-Llopis, S. et al. BAP1 loss defines a new class of renal cell carcinoma. Nat. Genet. 44, 751–759 (2012).
Duns, G. et al. Histone methyltransferase gene SETD2 is a novel tumor suppressor gene in clear cell renal cell carcinoma. Cancer Res. 70, 4287–4291 (2010).
Gossage, L. et al. Clinical and pathological impact of VHL, PBRM1, BAP1, SETD2, KDM6A, and JARID1c in clear cell renal cell carcinoma. Genes Chromosomes Cancer 53, 38–51 (2014).
McDonald, S. A. et al. Mechanisms of field cancerization in the human stomach: the expansion and spread of mutated gastric stem cells. Gastroenterology 134, 500–510 (2008).
Wright, N. A. Boveri at 100: cancer evolution, from preneoplasia to malignancy. J. Pathol. 234, 146–151 (2014).
Foschini, M. P. et al. Genetic clonal mapping of in situ and invasive ductal carcinoma indicates the field cancerization phenomenon in the breast. Hum. Pathol. 44, 1310–1319 (2013).
Dotto, G. P. Multifocal epithelial tumors and field cancerization: stroma as a primary determinant. J. Clin. Invest. 124, 1446–1453 (2014).
Fearon, E. R. & Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 61, 759–767 (1990).
Hou, J., Rajagopal, M. & Yu, A. S. Claudins and the kidney. Annu. Rev. Physiol. 75, 479–501 (2013).
Ramos, A. et al. The liver in autosomal dominant polycystic kidney disease. Implications for pathogenesis. Arch. Pathol. Lab. Med. 114, 180–184 (1990).
Yu, A. S. et al. Tight junction composition is altered in the epithelium of polycystic kidneys. J. Pathol. 216, 120–128 (2008).
Runkle, E. A. & Mu, D. Tight junction proteins: from barrier to tumorigenesis. Cancer Lett. 337, 41–48 (2013).
Kwon, M. J. Emerging roles of claudins in human cancer. Int. J. Mol. Sci. 14, 18148–18180 (2013).
Osunkoya, A. O. et al. Claudin-7 and claudin-8: immunohistochemical markers for the differential diagnosis of chromophobe renal cell carcinoma and renal oncocytoma. Hum. Pathol. 40, 206–210 (2009).
Melchers, L. J. et al. Lack of claudin-7 is a strong predictor of regional recurrence in oral and oropharyngeal squamous cell carcinoma. Oral Oncol. 49, 998–1005 (2013).
Bornholdt, J. et al. The level of claudin-7 is reduced as an early event in colorectal carcinogenesis. BMC Cancer 11, 65 (2011).
Grivennikov, S. I. et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 491, 254–258 (2012).
Choi, W. et al. Intrinsic basal and luminal subtypes of muscle-invasive bladder cancer. Nat. Rev. Urol. 11, 400–410 (2014).
Harten, S. K. et al. Regulation of renal epithelial tight junctions by the von Hippel–Lindau tumor suppressor gene involves occludin and claudin 1 and is independent of E-cadherin. Mol. Biol. Cell 20, 1089–1101 (2009).
De Nardo, D., De Nardo, C. M. & Latz, E. New insights into mechanisms controlling the NLRP3 inflammasome and its role in lung disease. Am. J. Pathol. 184, 42–54 (2014).
Chang, A., Ko, K. & Clark, M. R. The emerging role of the inflammasome in kidney diseases. Curr. Opin. Nephrol. Hypertens. 23, 204–210 (2014).
Settleman, J. Oncogene addiction. Curr. Biol. 22, R43–R44 (2012).
Sharma, S. V. & Settleman, J. Oncogene addiction: setting the stage for molecularly targeted cancer therapy. Genes Dev. 21, 3214–3231 (2007).
Delmore, J. E. et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 146, 904–917 (2011).
Liu, H. et al. MYC suppresses cancer metastasis by direct transcriptional silencing of αv and β3 integrin subunits. Nat. Cell Biol. 14, 567–574 (2012).
Takiar, V. et al. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc. Natl Acad. Sci. USA 108, 2462–2467 (2011).
Pernicova, I. & Korbonits, M. Metformin—mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol. 10, 143–156 (2014).
Dorff, T. B., Pal, S. K. & Quinn, D. I. Novel tyrosine kinase inhibitors for renal cell carcinoma. Expert Rev. Clin. Pharmacol. 7, 67–73 (2014).
Logan, T. F. Foretinib (XL880): c-MET inhibitor with activity in papillary renal cell cancer. Curr. Oncol. Rep. 15, 83–90 (2013).
American Cancer Society. Cancer Facts & Figures 2014. cancer.org [online], (2014).
Piccirillo, J. F. et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 291, 2441–2447 (2004).
Torres, V. E. et al. Angiotensin blockade in late autosomal dominant polycystic kidney disease. N. Engl. J. Med. 371, 2267–2276 (2014).
Schrier, R. W. et al. Blood pressure in early autosomal dominant polycystic kidney disease. N. Engl. J. Med. 371, 2255–2266 (2014).
Ellison, D. H. & Ingelfinger, J. R. A quest—halting the progression of autosomal dominant polycystic kidney disease. N. Engl. J. Med. 371, 2329–2331 (2014).
Abi Aad, S. et al. Hypertension induced by chemotherapeutic and immunosuppresive agents: A new challenge. Crit. Rev. Oncol. Hematol. 93, 28–35 (2015).
Hamet, P. Cancer and hypertension. An unresolved issue. Hypertension 28, 321–324 (1996).
Sanfilippo, K. M. et al. Hypertension and obesity and the risk of kidney cancer in 2 large cohorts of US men and women. Hypertension 63, 934–941 (2014).
Sipahi, I. et al. Meta-analysis of randomized controlled trials on effect of angiotensin-converting enzyme inhibitors on cancer risk. Am. J. Cardiol. 108, 294–301 (2011).
Piersol, G. M. Polycystic Disease of the Kidney. Trans. Am. Climatol. Clin. Assoc. 43, 221–231 (1927).
Rossetti, S. et al. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 18, 2143–2160 (2007).
Audrezet, M. P. et al. Autosomal dominant polycystic kidney disease: comprehensive mutation analysis of PKD1 and PKD2 in 700 unrelated patients. Hum. Mutat. 33, 1239–1250 (2012).
Cornec-Le Gall, E. et al. Type of PKD1 mutation influences renal outcome in ADPKD. J. Am. Soc. Nephrol. 24, 1006–1013 (2013).
Lara, P. N. Jr & Jonasch, E. (Eds) Kidney Cancer: Principles and Practice. (Springer–Verlag, 2012).
Linehan, W. M. Genetic basis of bilateral renal cancer: implications for evaluation and management. J. Clin. Oncol. 27, 3731–3733 (2009).
Dutcher, J. P. Recent developments in the treatment of renal cell carcinoma. Ther. Adv. Urol. 5, 338–353 (2013).
Acknowledgements
We apologize to those of our colleagues whose work we were unable to cite because of reference limits. We are very grateful to Dr James Calvet and Dr Jared Grantham (University of Kansas Medical Center, USA), who generously provided many useful suggestions and comments on the manuscript. We also thank the reviewers of this article for their valuable critiques. The authors were supported by funding from the PKD Foundation and DOD PRMRP W81XWH-12-1-0437/PR110518 (to E.A.G.), from the German Research Foundation SE2280/3-1, Köln Fortune 252/2013 and by the German Ministry of Science and Education (BMBF) as part of the MILES consortium (grant 01ZX1406) (to T.S.-N.), Pfizer independent grant #11703,561 (to D.M.G.), BE2212, SFB829, SFB832 (to T.B.), and by NIH Core Grant CA06927 (to Fox Chase Cancer Center).
Author information
Authors and Affiliations
Contributions
T.S.-N., D.M.G., A.S.N. and E.A.G. researched data for the article. T.S.-N., D.M.G., and E.A.G. wrote the article. T.B. and A.S.N. contributed to the discussion and helped edit the manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Seeger-Nukpezah, T., Geynisman, D., Nikonova, A. et al. The hallmarks of cancer: relevance to the pathogenesis of polycystic kidney disease. Nat Rev Nephrol 11, 515–534 (2015). https://doi.org/10.1038/nrneph.2015.46
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2015.46
This article is cited by
-
Prioritized polycystic kidney disease drug targets and repurposing candidates from pre-cystic and cystic mouse Pkd2 model gene expression reversion
Molecular Medicine (2023)
-
1-Indanone retards cyst development in ADPKD mouse model by stabilizing tubulin and down-regulating anterograde transport of cilia
Acta Pharmacologica Sinica (2023)
-
Loss of Pkd1 limits susceptibility to colitis and colorectal cancer
Oncogenesis (2023)
-
Repurposing Methotrexate in Dampening SARS-CoV2-S1-Mediated IL6 Expression: Lessons Learnt from Lung Cancer
Inflammation (2022)
-
Targeting the vasopressin type-2 receptor for renal cell carcinoma therapy
Oncogene (2020)